Acidic Patch Mutant H2BE105A,E113A Recombinant Nucleosome with Linker DNA, Biotinylated
{"url":"https://www.epicypher.com/products/nucleosomes/mutant-nucleosomes/acidic-patch-mutant-h2be105a-e113a-recombinant-nucleosome-with-linker-dna-biotinylated","add_this":[{"service":"facebook","annotation":""},{"service":"email","annotation":""},{"service":"print","annotation":""},{"service":"twitter","annotation":""},{"service":"linkedin","annotation":""}],"gtin":null,"id":1147,"bulk_discount_rates":[],"can_purchase":true,"meta_description":"Acidic patch mutant recombinant mononucleosomes (biotinylated H2AE61A), functionally validated with exceptional quality controls. Useful for chromatin binding, enzymatic, and structural studies.","category":["Nucleosomes","Nucleosomes/Mutant Nucleosomes","Nucleosomes/Mutant Nucleosomes/Acidic Patch Nucleosomes"],"AddThisServiceButtonMeta":"","main_image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/1147/1219/2021.05.05_acidic_patch_nuc_RGB_white_border_LS_icon_thumbnail_board_2__84943.1716971267.png?c=2","alt":"Acidic Patch Mutant H2BE105A,E113A Recombinant Nucleosome with Linker DNA, Biotinylated"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=1147","shipping":{"calculated":true},"num_reviews":0,"weight":"0.00 LBS","custom_fields":[{"id":"1314","name":"Pack Size","value":"50 µg"}],"sku":"16-2031","description":" <div class=\"product-general-info\">\n <ul class=\"product-general-info__list-left\">\n <li class=\"product-general-info__list-item\">\n <strong>Species: </strong>Human\n </li>\n <li class=\"product-general-info__list-item\">\n <strong>Source: </strong><em>E. coli</em> & synthetic DNA\n </li>\n </ul>\n <ul class=\"product-general-info__list-right\">\n <li class=\"product-general-info__list-item\">\n <strong>Tag: </strong>Biotinylated\n </li>\n <li class=\"product-general-info__list-item\">\n <strong>Molecular Weight: </strong>231,649 Da\n </li>\n </ul>\n </div>\n <div class=\"service_accordion product-droppdown\">\n <div class=\"container\">\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel current\">\n <h3 class=\"sub-title1\">Description</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description-specific\"\n >\n <p>\n The acidic patch is a negatively charged region of the nucleosome surface that serves as a conserved interaction hub for\n neighboring nucleosomes and nucleosome binding proteins, often via salt bridges with arginine anchors [1]. \n The acidic patch plays a critical role in chromatin condensation and chromatin remodeling [1-3]. \n Recombinant mononucleosomes containing acidic patch mutations can be used to study the biological functions of the acidic patch.\n </p>\n <p>\n H2BE105A,E113A Recombinant Nucleosome with Linker DNA consists of 199 base pairs of DNA wrapped around an \n octamer core of histone proteins (two each of H2A, H2B, H3.1, and H4) to form a nucleosome, the basic\n repeating unit of chromatin. The 5’ biotin-TEG DNA consists of a core 147 bp 601 nucleosome assembly sequence [4] \n flanked by 26 bp linker sequences as underlined below. Histone H2B contains a glutamate-to-alanine (E-to-A) substitution\n at positions 105 and 113 (H2BE105A, E113A). H2BE105 and H2BE113 both reside in H2B alphaC helix extension and are associated\n with nucleosome binding factors such as H4-N-terminal tail, LANA, RCC1, and HMGN2 [1]. H2BE105A,E113A disrupts binding with \n SMARCB1/BAF47, a subunit of the SWI/SNF (BAF) family of chromatin remodeling complexes. These complexes serve a critical role\n in cell division, cell and tissue differentiation, and development, and SWI/SNF complex malfunction has been linked to over \n 20% of human cancers [5].\n </p>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel current\">\n <h3 class=\"sub-title1\">Validation Data</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description-specific\"\n >\n <section class=\"image-picker\">\n <div class=\"image-picker__left\">\n <div\n class=\"image-picker__main-content_active image-picker__main-content\"\n >\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\"\n >\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\"\n />\n </svg>\n </button>\n <a\n href=\"/content/images/products/nucleosomes/16-2031-protein-gel-data-test.jpg\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\"\n ><img\n alt=\"16-0029-protein-gel-data-test\"\n src=\"/content/images/products/nucleosomes/16-2031-protein-gel-data-test.jpg\"\n class=\"image-picker__main-image\"\n />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n ></a\n >\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\"\n >\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\"\n />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\"\n ><strong>Figure 1: Protein gel data</strong><br />\n Coomassie stained SDS-PAGE gel of proteins in H2BE105A,E113A recombinant nucleosome (1 µg) \n demonstrates the purity of histones in the preparation. Sizes of molecular weight markers \n and positions of the core histones (H2A, H2BE105A,E113A, H3 and H4) are indicated.\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\"\n >\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\"\n />\n </svg>\n </button>\n <a\n href=\"/content/images/products/nucleosomes/16-2031-dna-gel-data-test.jpg\"\n \n target=\"_blank\"\n class=\"image-picker__main-image-link\"\n ><img\n alt=\"16-0029-dna-gel-data-test\"\n src=\"/content/images/products/nucleosomes/16-2031-dna-gel-data-test.jpg\"\n class=\"image-picker__main-image\"\n />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n ></a\n >\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\"\n >\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\"\n />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\"\n ><strong>Figure 2: DNA gel data </strong><br />\n H2BE105A,E113A recombinant nucleosome resolved by native PAGE and stained\n with ethidium bromide to visualize DNA.\n <strong>Lane 1:</strong> Free DNA (EpiCypher\n <a\n href=\"/products/nucleosomes/nucleosome-assembly-601-sequence-dna-199-bp-biotinylated\"\n target=\"_blank\"\n >18-2044</a\n >; 100 ng). Biotinylated DNA can dimerize (band at ~400 bp). <strong>Lane 2:</strong> Intact H2BE105A,E113A\n recombinant nucleosomes (400 ng).\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\"\n >\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\"\n />\n </svg>\n </button>\n <a\n href=\"/content/images/products/nucleosomes/16-2031-functional-binding-assay-test.jpg\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\"\n >\n <img\n alt=\"16-0029-functional-binding-assay-test\"\n src=\"/content/images/products/nucleosomes/16-2031-functional-binding-assay-test.jpg\"\n class=\"image-picker__main-image\"\n />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\"\n >\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\"\n />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong>Figure 3: Functional binding assay </strong><br />\n The presence of the acidic patch mutations disrupts LANA peptide binding to recombinant nucleosomes (WT control, EpiCypher \n <a\n href=\"https://www.epicypher.com/products/nucleosomes/mononucleosomes-recombinant-199x601-dna-biotinylated\"\n >16-2044</a\n >; H2AE61A, EpiCypher \n <a\n href=\"https://www.epicypher.com/products/nucleosomes/mutant-nucleosomes/acidic-patch-mutant-h2ae61a-recombinant-nucleosome-with-linker-dna-biotinylated\"\n >16-2029</a\n >; H2AE92K, EpiCypher\n <a\n href=\"/products/nucleosomes/mutant-nucleosomes/acidic-patch-mutant-h2ae92k-recombinant-nucleosome-with-linker-dna-biotinylated\"\n >16-2030</a\n >; H2BE105A,E113A, EpiCypher 16-2031). The binding of GST-tagged LANA peptide to biotinylated recombinant nucleosomes was assessed by AlphaLISA assay using Streptavidin Donor Beads and Glutathione Acceptor Beads (PerkinElmer). The presence of H2A acidic patch mutations completely blocks LANA binding, while H2B mutations cause a decrease in LANA binding affinity.\n </span>\n </p>\n </div>\n </div>\n <aside class=\"image-picker__right\">\n <div class=\"image-picker__gallery\">\n <img\n alt=\"16-0029-protein-gel-data-test\"\n src=\"/content/images/products/nucleosomes/16-2031-protein-gel-data-test.jpg\"\n width=\"200\"\n class=\"image-picker__side-image image-picker__side-image_active\"\n role=\"button\"\n />\n <img\n alt=\"16-0029-dna-gel-data-test\"\n src=\"/content/images/products/nucleosomes/16-2031-dna-gel-data-test.jpg\"\n class=\"image-picker__side-image\"\n role=\"button\"\n />\n <img\n alt=\"16-0029-functional-binding-assay-test\"\n src=\"/content/images/products/nucleosomes/16-2031-functional-binding-assay-test.jpg\"\n class=\"image-picker__side-image\"\n role=\"button\"\n />\n </div>\n </aside>\n </section>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Technical Information</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\"\n >\n <div class=\"product-tech-info\">\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>Storage</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n Stable for six months at -80°C from date of receipt. For best results, aliquot and avoid freeze/thaws.\n \n </div>\n </div>\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>Formulation</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n 10 mM Tris-HCl pH 7.5, 25 mM NaCl, 1 mM EDTA, 2 mM DTT, 20% glycerol (27.3 μg protein, 50 μg DNA + protein).\n </div>\n </div>\n </div>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Application Notes</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\"\n >\n <p>\n H2BE105A,E113A mononucleosome is highly purified and suitable for a variety of applications to test the effect of acidic patch mutation on enzymatic activity or chromatin binding. The biotinylated DNA enables affinity binding applications. For a corresponding unmodified control, we recommend EpiCypher \n <a href=\"https://www.epicypher.com/products/nucleosomes/mononucleosomes-recombinant-199x601-dna-biotinylated\" >16-2044</a>.\n </p>\n \n </div>\n </div>\n </div>\n \n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">DNA Sequence </h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\"\n >\n <p style=\"line-break: anywhere\">\n 5’Bio-TEG<br>\n <u>GGGACCCTATACGCGGCCGCCGAATTCC</u>TGGAGAATCCCGGTCTGCAGGCCGCTCAATTGGTCGTAGACAGCTCTAGCACCGCTTAAACGCACGTACGCGCTGTCCCCCGCGTTTTAACCGCCAAGGGGATTACTCCCTAGTCTCCAGGCACGTGTCAGATATATACATCCTGT<u>GGATCCGCCGGTCGCGAACAGCGACC</u>3’\n </p>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Gene & Protein Information</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\"\n >\n <div class=\"product-tech-info\">\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>UniProt ID</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n H2A - P04908 (alt. names: H2A type 1-B/E, H2A.2, H2A/a,\n H2A/m)<br />\n H2B - O60814 (alt. names: H2B K, HIRA-interacting protein 1)<br />\n H3.1 - P68431 (alt. names: H3, H3/a, H3/b, H3/c, H3/d)<br />\n H4 - P62805\n </div>\n </div>\n </div>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">References</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\"\n >\n <strong>Background References:</strong>\n <br />\n [1] Kalashnikova et al. <em>J. R. Soc. Interface</em> (2013). PMID: \n <a\n href=\"https://pubmed.ncbi.nlm.nih.gov/23446052/\"\n target=\"_blank\"\n title=\"New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning\"\n >23446052</a\n ><br />\n [2] Levendosky & Bowman <em>eLife</em> (2019). PMID: \n <a\n href=\"https://pubmed.ncbi.nlm.nih.gov/31094676/\"\n target=\"_blank\"\n title=\"The role of the nucleosome acidic patch in modulating higher order chromatin structure\"\n >31094676\n </a\n ><br />\n [3] Gamarra et al. <em>eLife</em> (2018). PMID: \n <a\n href=\"https://pubmed.ncbi.nlm.nih.gov/29664398/\"\n target=\"_blank\"\n title=\"Asymmetry between the two acidic patches dictates the direction of nucleosome sliding by the ISWI chromatin remodeler\"\n >29664398</a\n ><br />\n [4] Lowary & Widom. <em>J. Mol. Biol.</em> (1998). PMID: \n <a\n href=\"https://pubmed.ncbi.nlm.nih.gov/9514715/\"\n target=\"_blank\"\n title=\"The nucleosomal acidic patch relieves auto-inhibition by the ISWI remodeler SNF2h\"\n >9514715</a\n ><br />\n [5] Valencia et al. <em>Cell</em> (2019). PMID:\n <a\n href=\"https://pubmed.ncbi.nlm.nih.gov/31759698/\"\n target=\"_blank\"\n title=\"The nucleosomal acidic patch relieves auto-inhibition by the ISWI remodeler SNF2h\"\n >31759698</a\n ><br />\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Documents & Resources</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\"\n >\n <div class=\"product-documents\">\n <a\n href=\"/content/documents/tds/16-2031.pdf\"\n target=\"_blank\"\n class=\"product-documents__link\"\n >\n <svg\n version=\"1.1\"\n id=\"Layer_1\"\n xmlns=\"http://www.w3.org/2000/svg\"\n xmlns:xlink=\"http://www.w3.org/1999/xlink\"\n x=\"0px\"\n y=\"0px\"\n viewBox=\"0 0 228 240\"\n style=\"enable-background: new 0 0 228 240\"\n xml:space=\"preserve\"\n class=\"product-documents__icon\"\n alt=\"16-0029 Datasheet\"\n >\n <g>\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M191.92,68.77l-47.69-47.69c-1.33-1.33-3.12-2.08-5.01-2.08H45.09C41.17,19,38,22.17,38,26.09v184.36\n c0,3.92,3.17,7.09,7.09,7.09h141.82c3.92,0,7.09-3.17,7.09-7.09V73.8C194,71.92,193.25,70.1,191.92,68.77z M177.65,77.06h-41.7\n v-41.7L177.65,77.06z M178.05,201.59H53.95V34.95h66.92v47.86c0,5.14,4.17,9.31,9.31,9.31h47.86V201.59z\"\n />\n </g>\n <rect\n x=\"20\"\n y=\"112\"\n class=\"product-documents__svg-background\"\n width=\"146\"\n height=\"76\"\n />\n <g>\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M23.83,125.68h22.36c5.29,0,9.41,1.33,12.35,4c2.94,2.67,4.42,6.39,4.42,11.18c0,4.78-1.47,8.51-4.42,11.18\n c-2.94,2.67-7.06,4-12.35,4H34.59v18.29H23.83V125.68z M44.81,147.9c5.38,0,8.07-2.32,8.07-6.97c0-2.39-0.67-4.16-2-5.31\n c-1.33-1.15-3.36-1.73-6.07-1.73H34.59v14.01H44.81z\"\n />\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M69.92,125.68h18.91c5.29,0,9.84,0.97,13.66,2.9c3.82,1.93,6.74,4.72,8.76,8.35\n c2.02,3.63,3.04,7.98,3.04,13.04c0,5.06-1,9.42-3,13.08c-2,3.66-4.91,6.45-8.73,8.38c-3.82,1.93-8.4,2.9-13.73,2.9H69.92V125.68z\n M88.07,165.63c10.35,0,15.52-5.22,15.52-15.66c0-10.4-5.17-15.59-15.52-15.59h-7.38v31.26H88.07z\"\n />\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M122.57,125.68h32.84v8.49h-22.22v11.18h20.84v8.49h-20.84v20.49h-10.63V125.68z\"\n />\n </g>\n </svg>\n <span class=\"product-documents__info\">Technical Datasheet</span>\n </a>\n </div>\n </div>\n </div>\n </div>\n </div>\n </div>\n \n","tags":[],"warranty":"","price":{"without_tax":{"formatted":"$475.00","value":475,"currency":"USD"},"tax_label":"Sales Tax"},"detail_messages":"","availability":"","page_title":"Acidic Patch Mutant Recombinant Nucleosome | H2AE61A, Biotinylated","cart_url":"https://www.epicypher.com/cart.php","max_purchase_quantity":0,"mpn":null,"upc":null,"options":[],"related_products":[{"id":1148,"sku":"16-2029","name":"Acidic Patch Mutant H2AE61A Recombinant Nucleosome with Linker DNA, Biotinylated","url":"https://www.epicypher.com/products/nucleosomes/mutant-nucleosomes/acidic-patch-mutant-h2ae61a-recombinant-nucleosome-with-linker-dna-biotinylated","availability":"","rating":null,"brand":{"name":null},"category":["Nucleosomes","Nucleosomes/Mutant Nucleosomes","Nucleosomes/Mutant Nucleosomes/Acidic Patch Nucleosomes"],"summary":"\n \n \n Species: Human\n \n \n Source: E. coli & synthetic DNA\n \n \n \n ","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/1148/1221/2021.05.05_acidic_patch_nuc_RGB_white_border_LS_icon_thumbnail_board_2__41959.1717092961.png?c=2","alt":"Acidic Patch Mutant H2AE61A Recombinant Nucleosome with Linker DNA, Biotinylated"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/1148/1221/2021.05.05_acidic_patch_nuc_RGB_white_border_LS_icon_thumbnail_board_2__41959.1717092961.png?c=2","alt":"Acidic Patch Mutant H2AE61A Recombinant Nucleosome with Linker DNA, Biotinylated"}],"date_added":"30th May 2024","pre_order":false,"show_cart_action":true,"has_options":false,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":[{"id":1315,"name":"Pack Size","value":"50 µg"}],"num_reviews":null,"weight":{"formatted":"0.00 LBS","value":0},"demo":false,"add_to_cart_url":"https://www.epicypher.com/cart.php?action=add&product_id=1148","price":{"without_tax":{"currency":"USD","formatted":"$475.00","value":475},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=1148"},{"id":705,"sku":"16-2030","name":"Acidic Patch Mutant H2AE92K Recombinant Nucleosome with Linker DNA, Biotinylated","url":"https://www.epicypher.com/products/nucleosomes/mutant-nucleosomes/acidic-patch-mutant-h2ae92k-recombinant-nucleosome-with-linker-dna-biotinylated","availability":"","rating":null,"brand":{"name":null},"category":["Nucleosomes","Nucleosomes/Mutant Nucleosomes","Nucleosomes/Mutant Nucleosomes/Acidic Patch Nucleosomes"],"summary":"\n \n \n Species: Human\n \n \n Source: E. coli & synthetic DNA\n \n \n \n ","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/705/1227/2021.05.05_acidic_patch_nuc_RGB_white_border_LS_icon_thumbnail_board_2__41959__47696.1717694541.png?c=2","alt":"Acidic Patch Mutant H2AE92K Recombinant Nucleosome with Linker DNA, Biotinylated"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/705/1227/2021.05.05_acidic_patch_nuc_RGB_white_border_LS_icon_thumbnail_board_2__41959__47696.1717694541.png?c=2","alt":"Acidic Patch Mutant H2AE92K Recombinant Nucleosome with Linker DNA, Biotinylated"}],"date_added":"18th Oct 2019","pre_order":false,"show_cart_action":true,"has_options":false,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":[{"id":492,"name":"Pack size","value":"50 µg"}],"num_reviews":null,"weight":{"formatted":"0.01 LBS","value":0.01},"demo":false,"add_to_cart_url":"https://www.epicypher.com/cart.php?action=add&product_id=705","price":{"without_tax":{"currency":"USD","formatted":"$475.00","value":475},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=705"},{"id":792,"sku":"16-2044","name":"Mononucleosomes, Recombinant, 199x601 DNA, Biotinylated","url":"https://www.epicypher.com/products/nucleosomes/mononucleosomes-recombinant-199x601-dna-biotinylated","availability":"","rating":null,"brand":{"name":null},"category":["Nucleosomes","Nucleosomes/Methyl DNA Designer Nucleosomes"],"summary":"\n \n \n Species: Human\n \n \n Source: E. coli & synthetic DNA\n \n \n \n ","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/792/931/mononucleosomes-recombinant-199x601-dna-biotinylated__61382.1645734522.jpg?c=2","alt":"Mononucleosomes, Recombinant, 199x601 DNA, Biotinylated"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/792/931/mononucleosomes-recombinant-199x601-dna-biotinylated__61382.1645734522.jpg?c=2","alt":"Mononucleosomes, Recombinant, 199x601 DNA, Biotinylated"}],"date_added":"21st Oct 2020","pre_order":false,"show_cart_action":true,"has_options":true,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":[{"id":1187,"name":"Pack size","value":"50 µg"}],"num_reviews":null,"weight":{"formatted":"0.01 LBS","value":0.01},"demo":false,"price":{"without_tax":{"currency":"USD","formatted":"$575.00","value":575},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=792"},{"id":861,"sku":"16-0031","name":"Nucleosome, Recombinant Human, Acidic Patch Mutant H2BE105A,E113A Biotinylated","url":"https://www.epicypher.com/products/nucleosomes/mutant-nucleosomes/nucleosome-recombinant-human-acidic-patch-mutant-h2be105a-e113a-biotinylated","availability":"","rating":null,"brand":{"name":null},"category":["Nucleosomes/Mutant Nucleosomes","Nucleosomes/Mutant Nucleosomes/Acidic Patch Nucleosomes"],"summary":"\n \n \n Species: Human\n \n \n Source: E. coli & synthetic DNA\n \n \n \n ","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/861/927/nucleosome-recombinant-human-acidic-patch-mutant-h2be105ae113a-biotinylated__42717.1645734518.jpg?c=2","alt":"Nucleosome, Recombinant Human, Acidic Patch Mutant H2BE105A,E113A Biotinylated"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/861/927/nucleosome-recombinant-human-acidic-patch-mutant-h2be105ae113a-biotinylated__42717.1645734518.jpg?c=2","alt":"Nucleosome, Recombinant Human, Acidic Patch Mutant H2BE105A,E113A Biotinylated"},{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/861/885/2021.05.05_acidic_patch_nuc_RGB_LS__97220.1621279281.png?c=2","alt":"Nucleosome, Recombinant Human, Acidic Patch Mutant H2BE105A,E113A Biotinylated"}],"date_added":"17th May 2021","pre_order":false,"show_cart_action":true,"has_options":false,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":[{"id":806,"name":"Pack Size","value":"50 µg"}],"num_reviews":null,"weight":{"formatted":"0.00 LBS","value":0},"demo":false,"add_to_cart_url":"https://www.epicypher.com/cart.php?action=add&product_id=861","price":{"without_tax":{"currency":"USD","formatted":"$475.00","value":475},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=861"}],"shipping_messages":[],"rating":0,"meta_keywords":"acidic patch mutant, mutant nucleosome, mutant histone, H2AE61A","show_quantity_input":1,"title":"Acidic Patch Mutant H2BE105A,E113A Recombinant Nucleosome with Linker DNA, Biotinylated","gift_wrapping_available":false,"min_purchase_quantity":0,"customizations":[],"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/1147/1219/2021.05.05_acidic_patch_nuc_RGB_white_border_LS_icon_thumbnail_board_2__84943.1716971267.png?c=2","alt":"Acidic Patch Mutant H2BE105A,E113A Recombinant Nucleosome with Linker DNA, Biotinylated"}]} Pack Size: 50 µg
- Species: Human
- Source: E. coli & synthetic DNA
- Tag: Biotinylated
- Molecular Weight: 231,649 Da
Description
The acidic patch is a negatively charged region of the nucleosome surface that serves as a conserved interaction hub for neighboring nucleosomes and nucleosome binding proteins, often via salt bridges with arginine anchors [1]. The acidic patch plays a critical role in chromatin condensation and chromatin remodeling [1-3]. Recombinant mononucleosomes containing acidic patch mutations can be used to study the biological functions of the acidic patch.
H2BE105A,E113A Recombinant Nucleosome with Linker DNA consists of 199 base pairs of DNA wrapped around an octamer core of histone proteins (two each of H2A, H2B, H3.1, and H4) to form a nucleosome, the basic repeating unit of chromatin. The 5’ biotin-TEG DNA consists of a core 147 bp 601 nucleosome assembly sequence [4] flanked by 26 bp linker sequences as underlined below. Histone H2B contains a glutamate-to-alanine (E-to-A) substitution at positions 105 and 113 (H2BE105A, E113A). H2BE105 and H2BE113 both reside in H2B alphaC helix extension and are associated with nucleosome binding factors such as H4-N-terminal tail, LANA, RCC1, and HMGN2 [1]. H2BE105A,E113A disrupts binding with SMARCB1/BAF47, a subunit of the SWI/SNF (BAF) family of chromatin remodeling complexes. These complexes serve a critical role in cell division, cell and tissue differentiation, and development, and SWI/SNF complex malfunction has been linked to over 20% of human cancers [5].
Validation Data
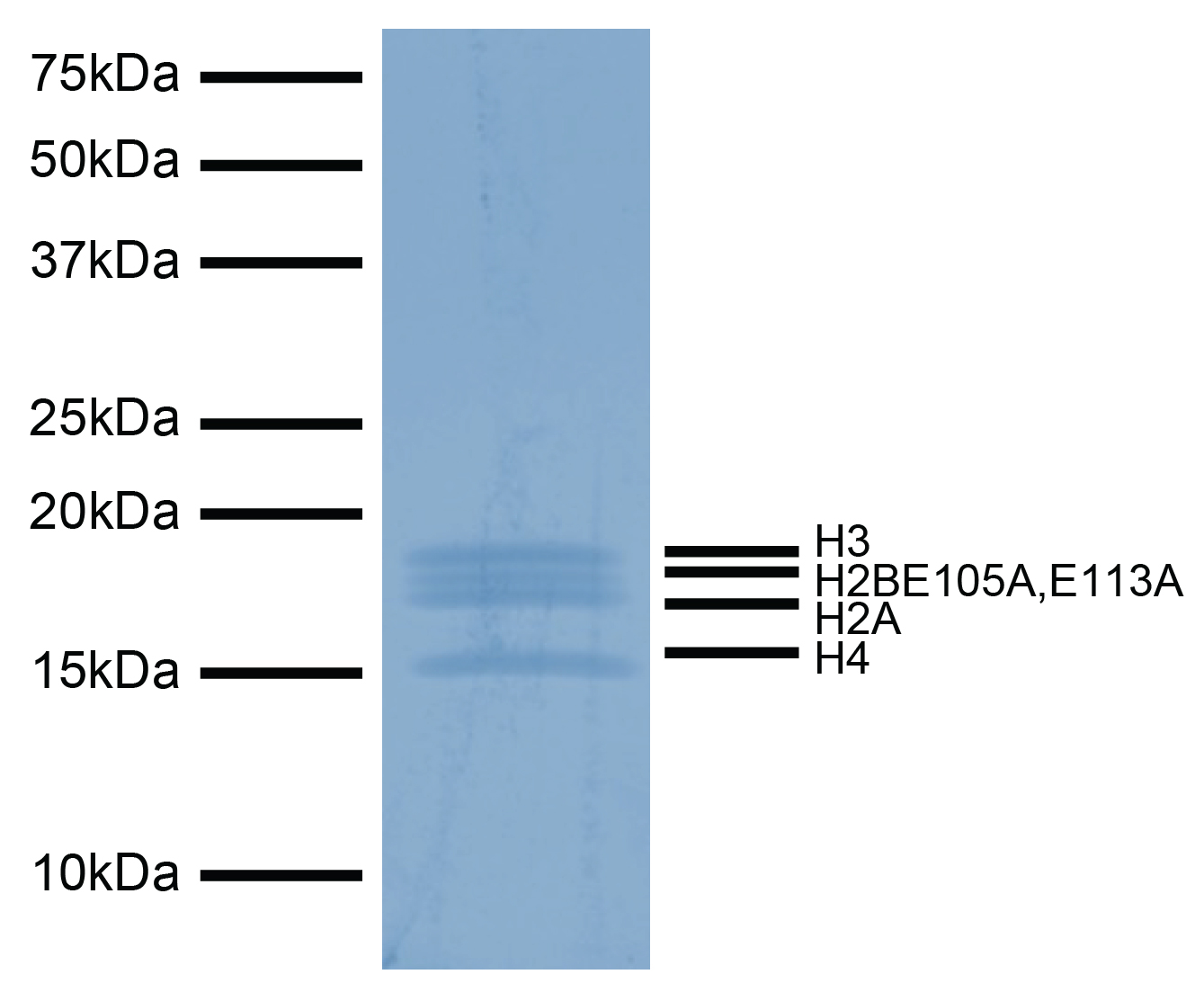
Figure 1: Protein gel data
Coomassie stained SDS-PAGE gel of proteins in H2BE105A,E113A recombinant nucleosome (1 µg)
demonstrates the purity of histones in the preparation. Sizes of molecular weight markers
and positions of the core histones (H2A, H2BE105A,E113A, H3 and H4) are indicated.
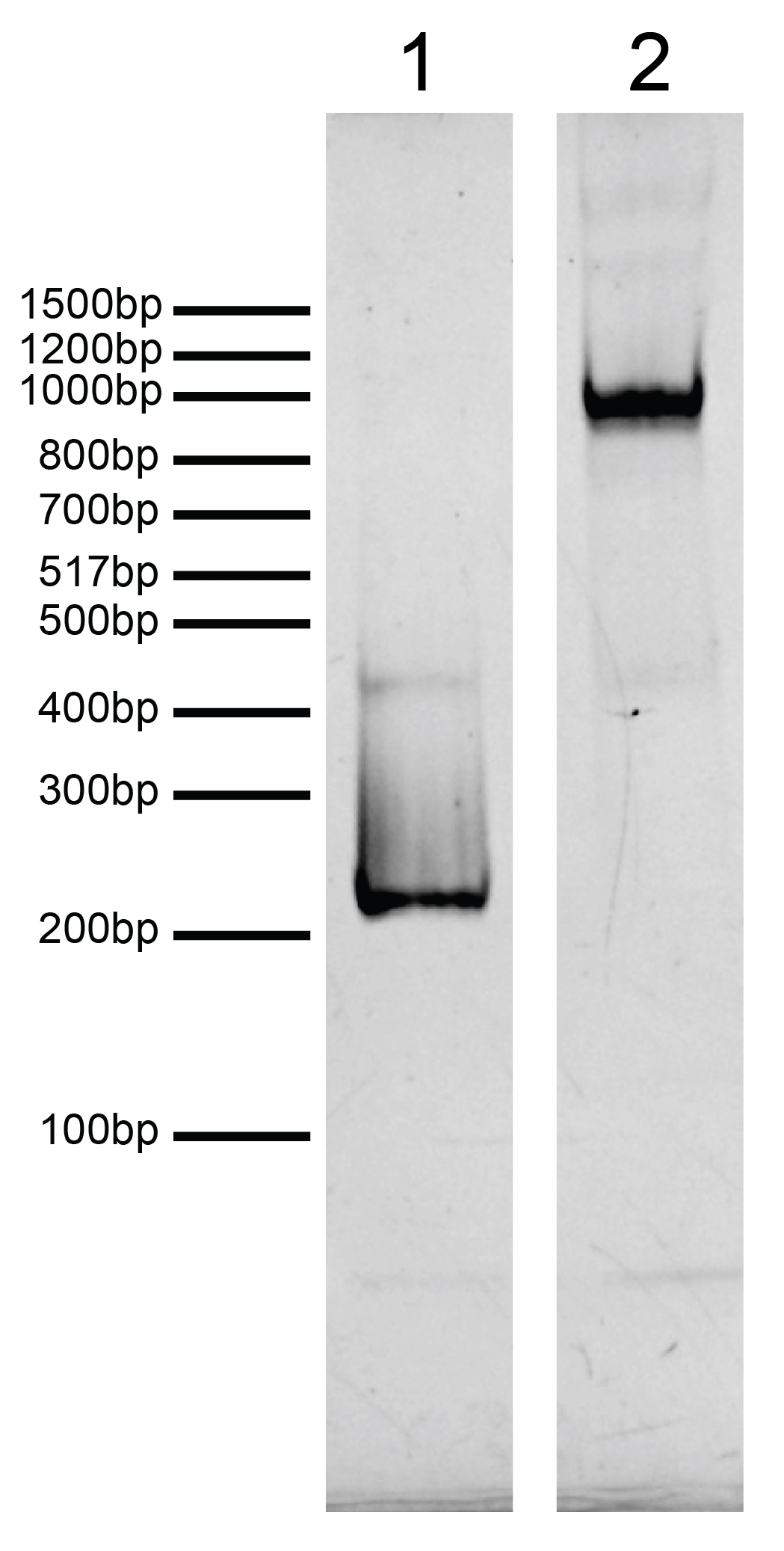
Figure 2: DNA gel data
H2BE105A,E113A recombinant nucleosome resolved by native PAGE and stained
with ethidium bromide to visualize DNA.
Lane 1: Free DNA (EpiCypher
18-2044; 100 ng). Biotinylated DNA can dimerize (band at ~400 bp). Lane 2: Intact H2BE105A,E113A
recombinant nucleosomes (400 ng).
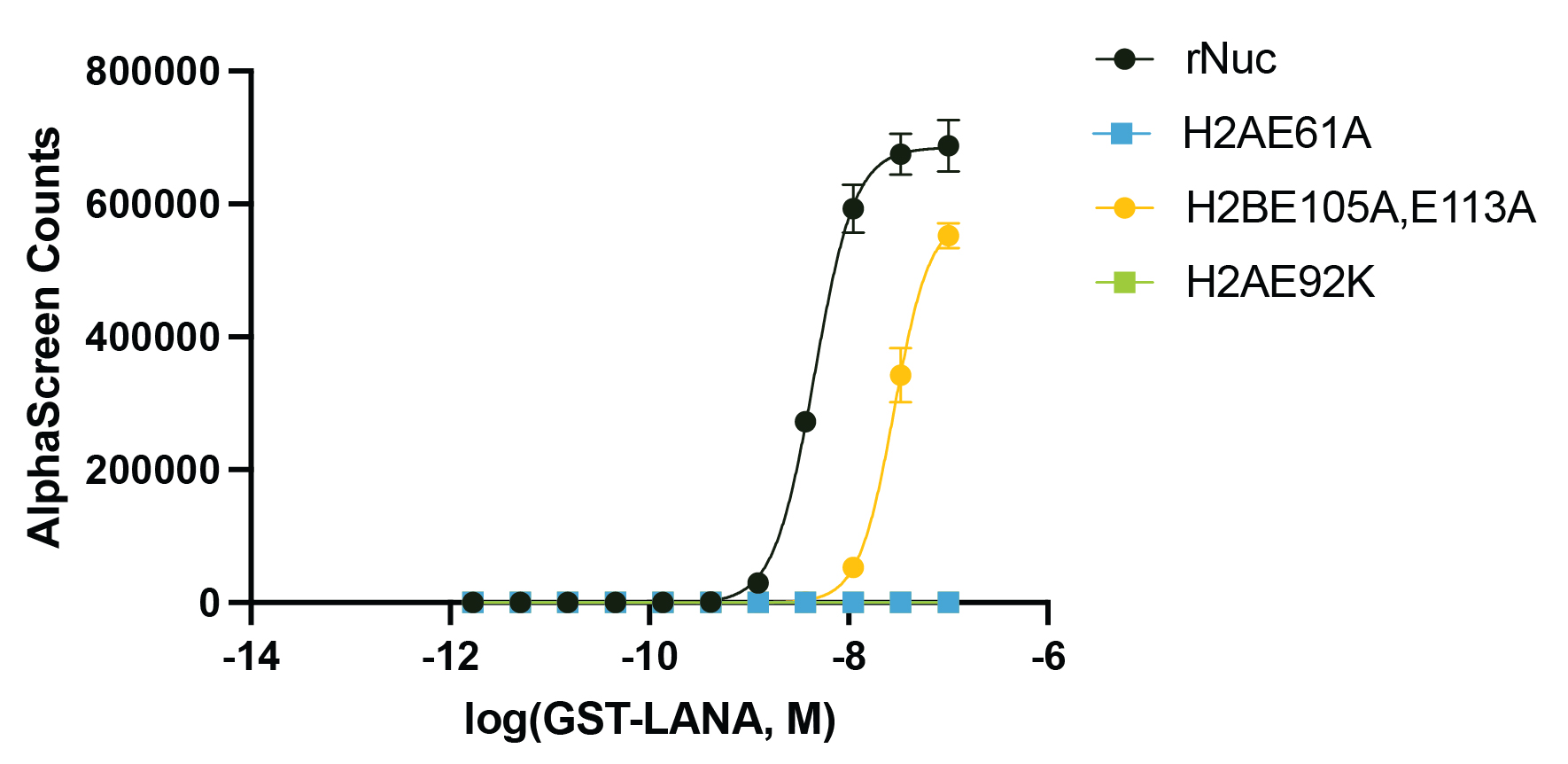
Figure 3: Functional binding assay
The presence of the acidic patch mutations disrupts LANA peptide binding to recombinant nucleosomes (WT control, EpiCypher
16-2044; H2AE61A, EpiCypher
16-2029; H2AE92K, EpiCypher
16-2030; H2BE105A,E113A, EpiCypher 16-2031). The binding of GST-tagged LANA peptide to biotinylated recombinant nucleosomes was assessed by AlphaLISA assay using Streptavidin Donor Beads and Glutathione Acceptor Beads (PerkinElmer). The presence of H2A acidic patch mutations completely blocks LANA binding, while H2B mutations cause a decrease in LANA binding affinity.
Technical Information
Application Notes
H2BE105A,E113A mononucleosome is highly purified and suitable for a variety of applications to test the effect of acidic patch mutation on enzymatic activity or chromatin binding. The biotinylated DNA enables affinity binding applications. For a corresponding unmodified control, we recommend EpiCypher 16-2044.
DNA Sequence
5’Bio-TEG
GGGACCCTATACGCGGCCGCCGAATTCCTGGAGAATCCCGGTCTGCAGGCCGCTCAATTGGTCGTAGACAGCTCTAGCACCGCTTAAACGCACGTACGCGCTGTCCCCCGCGTTTTAACCGCCAAGGGGATTACTCCCTAGTCTCCAGGCACGTGTCAGATATATACATCCTGTGGATCCGCCGGTCGCGAACAGCGACC3’
Gene & Protein Information
H2B - O60814 (alt. names: H2B K, HIRA-interacting protein 1)
H3.1 - P68431 (alt. names: H3, H3/a, H3/b, H3/c, H3/d)
H4 - P62805