Nucleosome narratives: SMARCAL1-mediated tumor immune evasion

Senior author Alberto Ciccia, PhD (left) and first author Giuseppe Leuzzi, PhD.
In this new blog series, we are sitting down with the lead scientists of recently published chromatin studies, providing a behind-the-scenes look into their work. At EpiCypher, we are interested in learning how chromatin technologies are integrated into different fields, such as immunology, cell & gene therapy, developmental biology, metabolism, and much more. What are the major conclusions of their research? How does the paper impact the epigenetics field?
For this blog, we interviewed Giuseppe Leuzzi, PhD, and Associate Professor Alberto Ciccia, PhD, from the Department of Genetics and Development at Columbia University Irving Medical Center, about their recent Cell paper entitled “SMARCAL1 is a dual regulator of innate immune signaling and PD-L1 expression that promotes tumor immune evasion.” Of note, this was an intensely collaborative study, with labs contributing from around the globe – including EpiCypher.
The Ciccia laboratory investigates the role of the DNA damage response (DDR) in genome integrity and cancer development. In this study, Giuseppe Leuzzi, the first author of the paper, developed a novel genetic screen using CRISPR-Cas9 to evaluate the impact of DDR factors and chromatin regulators on innate immune signaling and PD-L1 immune checkpoint expression. Utilizing this approach, Leuzzi and colleagues identified SMARCAL1 as a novel regulator of tumor immune evasion and a promising target for more effective immuno-oncological therapies.
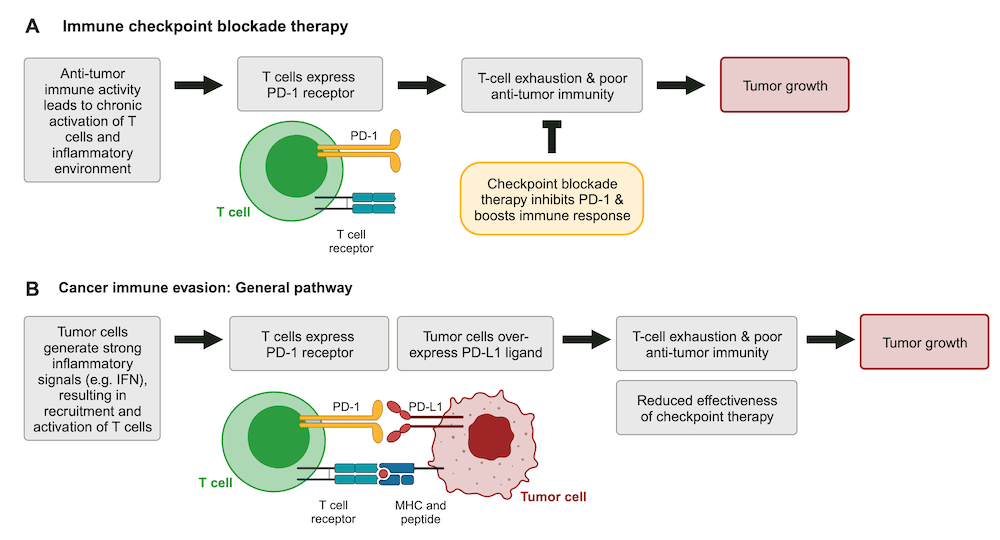
Background: Immune checkpoint blockade therapy and tumor resistance
The human immune system is designed to detect and destroy aberrant or infected cells, including rapidly proliferating tumor cells. Many cell types are involved in this process. For the purposes of this blog, we will focus on cytotoxic CD8+ T cells, which target and kill cancer cells.
In many cancer patients the immune system is chronically activated, leading to CD8+ T-cell exhaustion. Exhausted T cells upregulate various receptors, such as PD-1 and CTLA-4 receptors, which restrict T-cell proliferation, stimulation, and cytotoxic activity (Figure 1A). These signaling pathways reduce the effectiveness of CD8+ T cells in fighting cancer and are aptly referred to as immune checkpoints1-3.
Drugs that target immune checkpoint regulators represent a major breakthrough in cancer immunotherapy1. These treatments, such as the PD-1 inhibitor Keytruda (pembrolizumab), block immune checkpoints and help restore anti-tumor immune activity (Figure 1A). Unfortunately, cancers frequently find ways to co-opt checkpoint pathways or escape immunotherapy (Figure 1B). Understanding how tumor cells modulate these signals is critical to developing improved immunotherapies and biomarkers2.
How does the DNA damage response influence immune checkpoint therapy?
Genomic instability plays critical roles throughout cancer development, progression, and treatment response. Recent studies reveal that chemotherapy- and radiotherapy-induced DNA damage and DNA repair inhibition elevate interferon (IFN) expression in cancer cells. These innate immune signals can amplify the anti-tumor immune response by recruiting T cells to the tumor microenvironment, ultimately leading to tumor rejection. Thus, development of therapies that increase tumor-intrinsic IFN may be a useful strategy for cancer treatment.
However, prolonged IFN signaling creates the chronic inflammatory environment that drives T-cell exhaustion and immunosuppression1-3. In addition, IFN signaling can also result in PD-L1 over-expression in tumors – allowing tumor cells to evade the immune response as well as checkpoint therapy (Figure 1B).
Q&A with the authors
In Leuzzi et al., the authors explored novel mechanisms that regulate expression of PD-L1 and inflammatory cytokines in tumor cells. How can IFN signals be leveraged for enhanced immunotherapy? Their initial CRISPR screen indicated important roles for the SMARCAL1 DNA translocase protein in both pathways. A combination of genetically modified cell lines, mouse models, biochemical and epigenomics assays, and TCGA data were used to interrogate the function of SMARCAL1.
Below, Leuzzi and Ciccia discuss their work in the context of the field. Answers have been edited for brevity and clarity.
What is the take-home message from your paper?
We identified SMARCAL1 as a master regulator of cancer immune evasion and immunotherapy resistance. Specifically, SMARCAL1 acts in a tumor-intrinsic manner and allows tumor cells to evade both the endogenous immune system and therapies that block immune checkpoints.
Is that what you set out to study?
The study aimed to discover novel cancer drug targets that can strengthen the body's natural defense system while also countering immune evasion mechanisms, including tumor cell overexpression of the ligand PD-L1. The thought is that upregulation of innate immune signals (e.g. IFN) from cancer cells, with concomitant downregulation of immunosuppressive pathways, could restore sensitivity to immune checkpoint blockade therapy.
What were the most surprising results? Why?
We were most struck by the impact of SMARCAL1 deficiency, which induced cancer-autonomous IFN immune signaling while concurrently downregulating PD-L1 levels. This was very unexpected, given the conventional understanding that IFN signaling upregulates PD-L1 as part of the immune checkpoint response. This observation defies established expectations, revealing an unforeseen twist mediated by SMARCAL1 deficiency.
How does this work move the field forward?
This work advances the field by uncovering a previously unrecognized player, SMARCAL1, in tumor immune evasion. Notably, SMARCAL1 uses a unique dual mechanism to regulate IFN signals and PD-L1 levels. First, SMARCAL1 suppresses innate immune signaling by limiting endogenous DNA damage. Second, SMARCAL1 cooperates with JUN to induce PD-L1 expression, allowing cells to evade immune system surveillance. These findings open new avenues for targeted interventions that disrupt evasion mechanisms, potentially enhancing the effectiveness of cancer immunotherapy.
What essential insights did CUT&RUN and EpiDyne assays provide?
The EpiCypher team's expertise, coupled with the utilization of their CUT&RUN chromatin mapping and EpiDyne™ nucleosome remodeling techniques, played a crucial role in unraveling the molecular mechanism connecting SMARCAL1 to PD-L1 expression. CUT&RUN enabled precise mapping of SMARCAL1 binding sites, uncovering its collaboration with JUN at transcriptionally active chromatin regions. The EpiDyne assay contributed to evaluating SMARCAL1's capability in chromatin remodeling. Collectively, these techniques provided a comprehensive molecular characterization of how SMARCAL1 influences the chromatin landscape and regulates the PD-L1 immune checkpoint gene.
Summary and Conclusions
This exciting paper links the DNA damage response and chromatin regulation to tumor immune evasion and immunotherapy resistance, thereby opening new opportunities for the development of immuno-oncological treatments. Many thanks to Drs. Leuzzi and Ciccia for taking time to chat with us! We provided an overview here, and we strongly recommend reading their entire paper here.
We want to expand this series and speak with more scientists! How are you applying CUT&RUN, CUT&Tag, or other chromatin technologies to your area of research? Reach out to us and let us know.
References:
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12, 252-64 (2012). https://doi.org/10.1038/nrc3239.
- Fares CM et al. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am Soc Clin Oncol Educ Book 39, 147-64 (2019). https://doi.org/10.1200/EDBK_240837.
- Klapp V et al. The DNA Damage Response and Inflammation in Cancer. Cancer Discov 13, 1521-45 (2023). https://doi.org/10.1158/2159-8290.CD-22-1220
- Leuzzi G et al. SMARCAL1 is a dual regulator of innate immune signaling and PD-L1 expression that promotes tumor immune evasion. Cell (2024). https://doi.org/10.1016/j.cell.2024.01.008
