H3K4me1 Antibody, SNAP-Certified™ for CUT&RUN and CUT&Tag
{"sku":"13-0057","url":"https://www.epicypher.com/products/antibodies/cut-and-run-antibodies/cut-and-run-antibodies-histone-ptms/h3k4me1-antibody-snap-certified-for-cut-and-run-and-cut-and-tag","add_this":[{"service":"facebook","annotation":""},{"service":"email","annotation":""},{"service":"print","annotation":""},{"service":"twitter","annotation":""},{"service":"linkedin","annotation":""}],"gtin":null,"id":"985","bulk_discount_rates":[],"can_purchase":true,"meta_description":"Rabbit monoclonal histone H3K4me1 antibody rigorously tested for robust and reliable performance in CUT&RUN and CUT&Tag","category":["Antibodies/CUTANA™ CUT&RUN Antibodies","Antibodies/CUTANA™ CUT&RUN Antibodies/CUTANA™ CUT&RUN Antibodies to Histone PTMs","Antibodies/CUTANA™ CUT&Tag Antibodies","Epigenetics Kits and Reagents/CUTANA™ ChIC / CUT&RUN Assays","Epigenetics Kits and Reagents/CUTANA™ CUT&Tag Assays"],"AddThisServiceButtonMeta":"","main_image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/985/1115/snap-chip-ab__70507.1557259520.1280.1280__90586.1575483123.1280.1280__61997.1592491196__58196.1676557361__04967.1676559372.png?c=2","alt":"H3K4me1 Antibody, SNAP-Certified™ for CUT&RUN and CUT&Tag"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=985","shipping":{"calculated":true},"num_reviews":0,"weight":"0.01 LBS","description":"<div class=\"product-general-info\">\n <ul class=\"product-general-info__list-left\">\n <li class=\"product-general-info__list-item\">\n <strong>Type: </strong>Monoclonal [2088-1F4]\n </li>\n <li class=\"product-general-info__list-item\">\n <strong>Host: </strong>Rabbit\n </li>\n <li class=\"product-general-info__list-item\">\n <strong>Applications: </strong>CUT&RUN, CUT&Tag\n </li>\n </ul>\n <ul class=\"product-general-info__list-right\">\n <li class=\"product-general-info__list-item\">\n <strong>Reactivity: </strong>Human, Wide Range (Predicted)\n </li>\n <li class=\"product-general-info__list-item\">\n <strong>Format: </strong>Antigen affinity-purified\n </li>\n <li class=\"product-general-info__list-item\">\n <strong>Target Size: </strong>15 kDa\n </li>\n </ul>\n</div>\n\n<div class=\"service_accordion product-droppdown\">\n <div class=\"container\">\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel current\">\n <h3 class=\"sub-title1\">Description</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description-specific\">\n <p>\n This H3K4me1 (histone H3 lysine 4 monomethyl) antibody meets\n EpiCypher’s lot-specific SNAP-Certified™ criteria for specificity\n and efficient target enrichment in both CUT&RUN and CUT&Tag\n applications. This requires <20% cross-reactivity to related\n histone PTMs determined using the SNAP-CUTANA™ K-MetStat Panel of\n spike-in controls (EpiCypher\n <a href=\"/products/nucleosomes/snap-cutana-k-metstat-panel\"\n >19-1002</a\n >, <strong>Figures 1 and 4</strong>). High target efficiency is\n confirmed by consistent genomic enrichment at varying cell inputs:\n 500k and 50k cells in CUT&RUN (<strong>Figures 2-3</strong>); 100k\n and 10k cells in CUT&Tag (<strong>Figures 5-6</strong>). High\n efficiency antibodies display similar peak structures at\n representative loci (<strong>Figures 3 and 6</strong>) and highly\n conserved genome-wide signal (<strong>Figures 2 and 5</strong>) even\n at reduced cell numbers. H3K4me1 either flanks H3K4me3 at the\n transcription start site (TSS) or coincides with H3K4me3 (<strong\n >Figures 2-3, 5-6</strong\n >) [1].\n </p>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel current\">\n <h3 class=\"sub-title1\">Validation Data - CUT&RUN</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description-specific\">\n <section class=\"image-picker\">\n <div class=\"image-picker__left\">\n <div\n class=\"image-picker__main-content_active image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/antibodies/13-0057-specificity-analysis.jpeg\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\"\n ><img\n loading=\"lazy\"\n alt=\"13-0057-specificity-analysis\"\n src=\"/content/images/products/antibodies/13-0057-specificity-analysis.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n ></a\n >\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\"\n ><strong\n >Figure 1: SNAP specificity analysis in CUT&RUN </strong\n ><br />\n CUT&RUN was performed as described above. CUT&RUN sequencing\n reads were aligned to the unique DNA barcodes corresponding\n to each nucleosome in the K-MetStat panel (x-axis). Data are\n expressed as a percent relative to on-target recovery\n (H3K4me1 set to 100%).\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/antibodies/13-0057-cut-run-genome-wide-enrichment.jpeg\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\"\n ><img\n loading=\"lazy\"\n alt=\"13-0057-cut-run-genome-wide-enrichment\"\n src=\"/content/images/products/antibodies/13-0057-cut-run-genome-wide-enrichment.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n ></a\n >\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\"\n ><strong>Figure 2: CUT&RUN genome-wide enrichment</strong\n ><br />\n CUT&RUN was performed as described above. Sequence reads\n were aligned to 18,793 annotated transcription start sites\n (TSSs ± 2 kbp). Signal enrichment was sorted from highest to\n lowest (top to bottom) relative to the H3K4me1 - 500k cells\n sample (all gene rows aligned). High, medium, and low\n intensity are shown in red, yellow, and blue, respectively.\n H3K4me3 positive control and H3K4me1 antibodies produced the\n expected enrichment pattern, which was consistent between\n 500k and 50k cells and greater than the IgG negative\n control.\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/antibodies/13-0057-cut-run-browser-tracks.jpeg\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"13-0057-cut-run-browser-tracks\"\n src=\"/content/images/products/antibodies/13-0057-cut-run-browser-tracks.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong\n >Figure 3: H3K4me1 CUT&RUN representative browser\n tracks</strong\n ><br />\n CUT&RUN was performed as described above. Gene browser shots\n were generated using the Integrative Genomics Viewer (IGV,\n Broad Institute). H3K4me1 antibody tracks display the\n characteristic enrichment known to be consistent with the\n function of this PTM [1]. Similar results in peak structure\n and location were observed for both 500k and 50k cell\n inputs.\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/methods/cut-and-run-methods.png\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"cut-and-run-methods\"\n src=\"/content/images/products/methods/cut-and-run-methods.png\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong>CUT&RUN methods</strong><br />\n CUT&RUN was performed on 500k and 50k K562 cells with the\n SNAP-CUTANA™ K-MetStat Panel (EpiCypher\n <a href=\"/products/nucleosomes/snap-cutana-k-metstat-panel\"\n >19-1002</a\n >) spiked-in prior to the addition of 0.5 µg of either IgG\n negative control (EpiCypher\n <a\n href=\"/products/nucleosomes/snap-cutana-spike-in-controls/cutana-rabbit-igg-cut-run-negative-control-antibody\"\n >13-0042</a\n >), H3K4me3 positive control (EpiCypher\n <a\n href=\"/products/antibodies/snap-chip-certified-antibodies/histone-h3k4me3-antibody-snap-chip-certified-cutana-cut-run-compatible\"\n >13-0041</a\n >), or H3K4me1 antibodies. The experiment was performed\n using the CUTANA™ ChIC/CUT&RUN Kit v3.0 (EpiCypher\n <a\n href=\"/products/epigenetics-reagents-and-assays/cutana-chic-cut-and-run-kit\"\n >14-1048</a\n >). Library preparation was performed with 5 ng of CUT&RUN\n enriched DNA (or the total amount recovered if less than 5\n ng) using the CUTANA™ CUT&RUN Library Prep Kit (EpiCypher\n <a\n href=\"/products/epigenetics-reagents-and-assays/cutana-cut-and-run-library-prep-kit\"\n >14-1001/14-1002</a\n >). Both kit protocols were adapted for high throughput\n Tecan liquid handling. Libraries were run on an Illumina\n NextSeq2000 with paired-end sequencing (2x50 bp). Sample\n sequencing depth was 6.7 million reads (IgG 50k cell input),\n 11.5 million reads (IgG 500k cell input), 10.2 million reads\n (H3K4me3 50k cell input) and 16.7 million reads (H3K4me3\n 500k cell input). Data were aligned to the hg19 genome using\n Bowtie2. Data were filtered to remove duplicates,\n multi-aligned reads, and ENCODE DAC Exclusion List regions.\n </span>\n </p>\n </div>\n </div>\n <aside class=\"image-picker__right\">\n <div class=\"image-picker__gallery\">\n <img\n loading=\"lazy\"\n alt=\"13-0057-specificity-analysis\"\n src=\"/content/images/products/antibodies/13-0057-specificity-analysis.jpeg\"\n width=\"200\"\n class=\"image-picker__side-image image-picker__side-image_active\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"13-0057-cut-run-genome-wide-enrichment\"\n src=\"/content/images/products/antibodies/13-0057-cut-run-genome-wide-enrichment.jpeg\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"13-0057-cut-run-browser-tracks\"\n src=\"/content/images/products/antibodies/13-0057-cut-run-browser-tracks.jpeg\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"cut-and-run-methods\"\n src=\"/content/images/products/methods/cut-and-run-methods.png\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n </div>\n </aside>\n </section>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Validation Data - CUT&Tag</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <section class=\"image-picker\">\n <div class=\"image-picker__left\">\n <div\n class=\"image-picker__main-content_active image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/antibodies/13-0057-cut-tag-specificity-analysis.jpeg\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"13-0057-cut-tag-specificity-analysis\"\n src=\"/content/images/products/antibodies/13-0057-cut-tag-specificity-analysis.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong\n >Figure 4: SNAP specificity analysis in CUT&Tag </strong\n ><br />\n CUT&Tag was performed as described above. CUT&Tag sequencing\n reads were aligned to the unique DNA barcodes corresponding\n to each nucleosome in the K-MetStat panel (x-axis). Data are\n expressed as a percent relative to on-target recovery\n (H3K4me1 set to 100%).\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/antibodies/13-0057-genome-wide-enrichment.jpeg\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"13-0057-genome-wide-enrichment\"\n src=\"/content/images/products/antibodies/13-0057-genome-wide-enrichment.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong>Figure 5: CUT&Tag genome-wide enrichment </strong\n ><br />\n CUT&Tag was performed as described above. Sequence reads\n were aligned to 18,793 annotated transcription start sites\n (TSSs ± 2 kbp). Signal enrichment was sorted from highest to\n lowest (top to bottom) relative to the H3K4me1 - 100k nuclei\n sample (all gene rows aligned). High, medium, and low\n intensity are shown in red, yellow, and blue, respectively.\n H3K4me3 positive control and H3K4me1 antibodies produced the\n expected enrichment pattern, which was consistent between\n 100k and 10k nuclei and greater than the IgG negative\n control.\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/antibodies/13-0057-representative-browser-tracks.jpeg\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"13-0057-representative-browser-tracks\"\n src=\"/content/images/products/antibodies/13-0057-representative-browser-tracks.jpeg\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong\n >Figure 6: H3K4me1 CUT&Tag representative browser tracks </strong\n ><br />\n CUT&Tag was performed as described above. Gene browser shots\n were generated using the Integrative Genomics Viewer (IGV,\n Broad Institute). H3K4me1 antibody tracks display the\n characteristic enrichment known to be consistent with the\n function of this PTM [1]. Similar results in peak structure\n and location were observed for both 100k and 10k nuclei\n inputs.\n </span>\n </p>\n </div>\n <div class=\"image-picker__main-content\">\n <div class=\"image-picker__header-content\">\n <button class=\"image-picker__left-arrow\">\n <svg\n class=\"image-picker__svg-left\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M16.67 0l2.83 2.829-9.339 9.175 9.339 9.167-2.83 2.829-12.17-11.996z\" />\n </svg>\n </button>\n <a\n href=\"/content/images/products/methods/cut-and-tag-methods.png\"\n target=\"_blank\"\n class=\"image-picker__main-image-link\">\n <img\n loading=\"lazy\"\n alt=\"cut-and-tag-methods\"\n src=\"/content/images/products/methods/cut-and-tag-methods.png\"\n class=\"image-picker__main-image\" />\n <span class=\"image-picker__main-image-caption\"\n >(Click to enlarge)</span\n >\n </a>\n <button class=\"image-picker__right-arrow\">\n <svg\n class=\"image-picker__svg-right\"\n width=\"24\"\n height=\"24\"\n viewBox=\"0 0 24 24\">\n <path\n d=\"M7.33 24l-2.83-2.829 9.339-9.175-9.339-9.167 2.83-2.829 12.17 11.996z\" />\n </svg>\n </button>\n </div>\n <p>\n <span class=\"image-picker__span-content\">\n <strong>CUT&Tag methods</strong><br />\n CUT&Tag was performed on 100k and 10k K562 nuclei with the\n SNAP-CUTANA™ K-MetStat Panel (EpiCypher\n <a href=\"/products/nucleosomes/snap-cutana-k-metstat-panel\"\n >19-1002</a\n >) spiked-in prior to the addition of 0.5 µg of either IgG\n negative control (EpiCypher\n <a\n href=\"/products/nucleosomes/snap-cutana-spike-in-controls/cutana-rabbit-igg-cut-run-negative-control-antibody\"\n >13-0042</a\n >), H3K4me3 positive control (EpiCypher\n <a\n href=\"/products/antibodies/snap-chip-certified-antibodies/histone-h3k4me3-antibody-snap-chip-certified-cutana-cut-run-compatible\"\n >13-0041</a\n >), or H3K4me1 antibodies. The experiment was performed\n using the CUTANA™ Direct-to-PCR CUT&Tag\n <a href=\"/protocols\">Protocol</a>. Libraries were run on an\n Illumina NextSeq2000 with paired-end sequencing (2x50 bp).\n Sample sequencing depth was 16.8 million reads (IgG 500k\n cell input), 14.4 million reads (H3K4me3 500k cell input),\n 26.2 million reads (H3K4me1 500k cell input) and 11.4\n million reads (H3K4me1 50k cell input). Data were aligned to\n the hg19 genome using Bowtie2. Data were filtered to remove\n duplicates, multi-aligned reads, and ENCODE DAC Exclusion List regions.\n </span>\n </p>\n </div>\n </div>\n <aside class=\"image-picker__right\">\n <div class=\"image-picker__gallery\">\n <img\n loading=\"lazy\"\n alt=\"13-0057-cut-tag-specificity-analysis\"\n src=\"/content/images/products/antibodies/13-0057-cut-tag-specificity-analysis.jpeg\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"13-0057-genome-wide-enrichment\"\n src=\"/content/images/products/antibodies/13-0057-genome-wide-enrichment.jpeg\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"13-0057-representative-browser-tracks\"\n src=\"/content/images/products/antibodies/13-0057-representative-browser-tracks.jpeg\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n <img\n loading=\"lazy\"\n alt=\"cut-and-tag-methods\"\n src=\"/content/images/products/methods/cut-and-tag-methods.png\"\n class=\"image-picker__side-image\"\n role=\"button\" />\n </div>\n </aside>\n </section>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Technical Information</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <div class=\"product-tech-info\">\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>Immunogen</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n A synthetic peptide corresponding to histone H3 monomethylated\n at lysine 4\n </div>\n </div>\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>Storage</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n Stable for 1 year at 4°C from date of receipt\n </div>\n </div>\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>Formulation</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n Antigen affinity-purified recombinant monoclonal antibody in Borate buffered saline pH\n 8.0, 0.09% sodium azide\n </div>\n </div>\n </div>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Recommended Dilution</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <div class=\"product-tech-info\">\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>CUT&RUN:</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n 0.5 µg per reaction\n </div>\n </div>\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>CUT&Tag:</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n 0.5 µg per reaction\n </div>\n </div>\n </div>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Gene & Protein Information</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <div class=\"product-tech-info\">\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>UniProt ID</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n H3.1 - P68431\n </div>\n </div>\n <div class=\"product-tech-info__line-item\">\n <div class=\"product-tech-info__line-item-left\">\n <b>Alternate Names</b>\n </div>\n <div class=\"product-tech-info__line-item-right\">\n H3, H3/a, H3/b, H3/c, H3/d\n </div>\n </div>\n </div>\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">References</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <strong>Background References:</strong>\n <br />\n [1] Bae & Lesch <em>Front Cell Dev. Biol.</em> (2020). PMID:\n <a\n href=\"https://pubmed.ncbi.nlm.nih.gov/32432110/\"\n target=\"_blank\"\n title=\"H3K4me1 Distribution Predicts Transcription State and Poising at Promoters\"\n >32432110</a\n ><br />\n </div>\n </div>\n </div>\n <div id=\"prodAccordion\">\n <div id=\"ProductDescription\" class=\"Block Panel\">\n <h3 class=\"sub-title1\">Documents & Resources</h3>\n <div\n class=\"ProductDescriptionContainer product-droppdown__section-description\">\n <div class=\"product-documents\">\n <a\n href=\"/content/documents/tds/13-0057.pdf\"\n target=\"_blank\"\n class=\"product-documents__link\">\n <svg\n version=\"1.1\"\n id=\"Layer_1\"\n xmlns=\"http://www.w3.org/2000/svg\"\n xmlns:xlink=\"http://www.w3.org/1999/xlink\"\n x=\"0px\"\n y=\"0px\"\n viewBox=\"0 0 228 240\"\n style=\"enable-background: new 0 0 228 240\"\n xml:space=\"preserve\"\n class=\"product-documents__icon\"\n alt=\"16-0030 Datasheet\">\n <g>\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M191.92,68.77l-47.69-47.69c-1.33-1.33-3.12-2.08-5.01-2.08H45.09C41.17,19,38,22.17,38,26.09v184.36\n c0,3.92,3.17,7.09,7.09,7.09h141.82c3.92,0,7.09-3.17,7.09-7.09V73.8C194,71.92,193.25,70.1,191.92,68.77z M177.65,77.06h-41.7\n v-41.7L177.65,77.06z M178.05,201.59H53.95V34.95h66.92v47.86c0,5.14,4.17,9.31,9.31,9.31h47.86V201.59z\" />\n </g>\n <rect\n x=\"20\"\n y=\"112\"\n class=\"product-documents__svg-background\"\n width=\"146\"\n height=\"76\" />\n <g>\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M23.83,125.68h22.36c5.29,0,9.41,1.33,12.35,4c2.94,2.67,4.42,6.39,4.42,11.18c0,4.78-1.47,8.51-4.42,11.18\n c-2.94,2.67-7.06,4-12.35,4H34.59v18.29H23.83V125.68z M44.81,147.9c5.38,0,8.07-2.32,8.07-6.97c0-2.39-0.67-4.16-2-5.31\n c-1.33-1.15-3.36-1.73-6.07-1.73H34.59v14.01H44.81z\" />\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M69.92,125.68h18.91c5.29,0,9.84,0.97,13.66,2.9c3.82,1.93,6.74,4.72,8.76,8.35\n c2.02,3.63,3.04,7.98,3.04,13.04c0,5.06-1,9.42-3,13.08c-2,3.66-4.91,6.45-8.73,8.38c-3.82,1.93-8.4,2.9-13.73,2.9H69.92V125.68z\n M88.07,165.63c10.35,0,15.52-5.22,15.52-15.66c0-10.4-5.17-15.59-15.52-15.59h-7.38v31.26H88.07z\" />\n <path\n class=\"product-documents__svg-pdf\"\n d=\"M122.57,125.68h32.84v8.49h-22.22v11.18h20.84v8.49h-20.84v20.49h-10.63V125.68z\" />\n </g>\n </svg>\n <span class=\"product-documents__info\">Technical Datasheet</span>\n </a>\n </div>\n </div>\n </div>\n </div>\n </div>\n</div>\n","tags":[],"warranty":"","price":{"without_tax":{"formatted":"$525.00","value":525,"currency":"USD"},"tax_label":"Sales Tax"},"detail_messages":"","availability":"","page_title":"H3K4me1 Antibody | SNAP-Certified for CUT&RUN and CUT&Tag","cart_url":"https://www.epicypher.com/cart.php","max_purchase_quantity":0,"mpn":null,"upc":null,"options":[],"related_products":[{"id":983,"sku":"13-0055","name":"H3K27me3 Antibody, SNAP-Certified™ for CUT&RUN and CUT&Tag","url":"https://www.epicypher.com/products/antibodies/h3k27me3-antibody-snap-certified-for-cut-run-and-cut-and-tag","availability":"","rating":null,"brand":{"name":null},"category":["Nucleosomes/SNAP-CUTANA™ Spike-in Controls","Antibodies","Antibodies/CUTANA™ CUT&RUN Antibodies","Antibodies/CUTANA™ CUT&RUN Antibodies/CUTANA™ CUT&RUN Antibodies to Histone PTMs","Antibodies/CUTANA™ CUT&Tag Antibodies","Epigenetics Kits and Reagents/CUTANA™ ChIC / CUT&RUN Assays","Epigenetics Kits and Reagents/CUTANA™ CUT&Tag Assays"],"summary":"\n \n \n Type: Monoclonal [2084-1G5]\n \n \n Host: Rabbit\n \n \n Applications: ","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/983/1113/snap-chip-ab__70507.1557259520.1280.1280__90586.1575483123.1280.1280__88802.1676322308.png?c=2","alt":"H3K27me3 Antibody, SNAP-Certified™ for CUT&RUN and CUT&Tag"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/983/1113/snap-chip-ab__70507.1557259520.1280.1280__90586.1575483123.1280.1280__88802.1676322308.png?c=2","alt":"H3K27me3 Antibody, SNAP-Certified™ for CUT&RUN and CUT&Tag"}],"date_added":"13th Feb 2023","pre_order":false,"show_cart_action":true,"has_options":false,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":[{"id":1217,"name":"Pack Size","value":"100 µg"},{"id":1218,"name":"Internal Comment","value":"extra box in top shelf of Venom"}],"num_reviews":null,"weight":{"formatted":"0.01 LBS","value":0.01},"demo":false,"add_to_cart_url":"https://www.epicypher.com/cart.php?action=add&product_id=983","price":{"without_tax":{"currency":"USD","formatted":"$525.00","value":525},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=983"},{"id":893,"sku":"13-0054","name":"Histone H4K20me3 Antibody, SNAP-Certified™ for CUT&RUN","url":"https://www.epicypher.com/products/antibodies/cut-and-run-antibodies/cut-and-run-antibodies-histone-ptms/histone-h4k20me3-antibody-snap-certified-for-cut-and-run","availability":"","rating":null,"brand":{"name":null},"category":["Antibodies/CUTANA™ CUT&RUN Antibodies","Antibodies/CUTANA™ CUT&RUN Antibodies/CUTANA™ CUT&RUN Antibodies to Histone PTMs","Epigenetics Kits and Reagents/CUTANA™ ChIC / CUT&RUN Assays"],"summary":"\n \n \n Type: Monoclonal\n \n \n Target Size: 15 kDa\n \n \n Format: Affi","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/893/923/1628650844.190.250__22739.1628695556__57006.1645069385.png?c=2","alt":"Histone H4K20me3 Antibody, SNAP-Certified™ for CUT&RUN"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/893/923/1628650844.190.250__22739.1628695556__57006.1645069385.png?c=2","alt":"Histone H4K20me3 Antibody, SNAP-Certified™ for CUT&RUN"}],"date_added":"17th Feb 2022","pre_order":false,"show_cart_action":true,"has_options":false,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":[{"id":989,"name":"Pack Size","value":"100 µg"}],"num_reviews":null,"weight":{"formatted":"0.01 LBS","value":0.01},"demo":false,"add_to_cart_url":"https://www.epicypher.com/cart.php?action=add&product_id=893","price":{"without_tax":{"currency":"USD","formatted":"$525.00","value":525},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=893"},{"id":625,"sku":"13-0027","name":"Histone H3K4me2 Antibody, SNAP-Certified™ for CUT&RUN","url":"https://www.epicypher.com/products/antibodies/cutana-cut-run-antibodies/cut-run-antibodies-histone-ptms/histone-h3k4me2-antibody-snap-certified-for-cut-run","availability":"","rating":null,"brand":{"name":null},"category":["Antibodies/CUTANA™ CUT&RUN Antibodies","Antibodies/CUTANA™ CUT&RUN Antibodies/CUTANA™ CUT&RUN Antibodies to Histone PTMs","Epigenetics Kits and Reagents/CUTANA™ ChIC / CUT&RUN Assays"],"summary":"\n \n \n Type: Monoclonal\n \n \n Target Size: 15 kDa\n \n \n Format: Affi","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/625/577/Screen_Shot_2018-09-25_at_2.00.27_PM__04625.1538135192.png?c=2","alt":"Histone H3K4me2 Antibody, SNAP-Certified™ for CUT&RUN"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/625/577/Screen_Shot_2018-09-25_at_2.00.27_PM__04625.1538135192.png?c=2","alt":"Histone H3K4me2 Antibody, SNAP-Certified™ for CUT&RUN"}],"date_added":"24th Sep 2018","pre_order":false,"show_cart_action":true,"has_options":false,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":[{"id":451,"name":"Pack Size","value":"100 μg"}],"num_reviews":null,"weight":{"formatted":"0.00 LBS","value":0},"demo":false,"add_to_cart_url":"https://www.epicypher.com/cart.php?action=add&product_id=625","price":{"without_tax":{"currency":"USD","formatted":"$525.00","value":525},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=625"},{"id":984,"sku":"13-0058","name":"H3K36me3 Antibody, SNAP-Certified™ for CUT&RUN","url":"https://www.epicypher.com/products/antibodies/cut-and-run-antibodies/cut-and-run-antibodies-histone-ptms/h3k36me3-antibody-snap-certified-for-cut-and-run","availability":"","rating":null,"brand":{"name":null},"category":["Antibodies/CUTANA™ CUT&RUN Antibodies","Epigenetics Kits and Reagents/CUTANA™ ChIC / CUT&RUN Assays"],"summary":"\n \n \n Type: Monoclonal [2091-1E2]\n \n \n Host: Rabbit\n \n \n Applications: ","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/984/1114/snap-chip-ab__70507.1557259520.1280.1280__90586.1575483123.1280.1280__61997.1592491196__58196.1676557361.png?c=2","alt":"H3K36me3 Antibody, SNAP-Certified™ for CUT&RUN"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/984/1114/snap-chip-ab__70507.1557259520.1280.1280__90586.1575483123.1280.1280__61997.1592491196__58196.1676557361.png?c=2","alt":"H3K36me3 Antibody, SNAP-Certified™ for CUT&RUN"}],"date_added":"16th Feb 2023","pre_order":false,"show_cart_action":true,"has_options":false,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":null,"num_reviews":null,"weight":{"formatted":"0.01 LBS","value":0.01},"demo":false,"add_to_cart_url":"https://www.epicypher.com/cart.php?action=add&product_id=984","price":{"without_tax":{"currency":"USD","formatted":"$525.00","value":525},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=984"},{"id":760,"sku":"13-0041","name":"H3K4me3 Antibody, SNAP-Certified™ for CUT&RUN","url":"https://www.epicypher.com/products/antibodies/snap-chip-certified-antibodies/histone-h3k4me3-antibody-snap-chip-certified-cutana-cut-run-compatible","availability":"","rating":null,"brand":{"name":null},"category":["Nucleosomes/SNAP-CUTANA™ Spike-in Controls","Antibodies","Antibodies/CUTANA™ CUT&RUN Antibodies","Antibodies/CUTANA™ CUT&RUN Antibodies/CUTANA™ CUT&RUN Antibodies to Histone PTMs","Epigenetics Kits and Reagents/CUTANA™ ChIC / CUT&RUN Assays"],"summary":"\n \n Type: Mixed Monoclonal*\n Host: Rabbit\n Applications: CUT&RUN, ICC/IF, WB ","image":{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/760/743/snap-chip-ab__70507.1557259520.1280.1280__90586.1575483123.1280.1280__61997.1592491196.png?c=2","alt":"H3K4me3 Antibody, SNAP-Certified™ for CUT&RUN"},"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/760/743/snap-chip-ab__70507.1557259520.1280.1280__90586.1575483123.1280.1280__61997.1592491196.png?c=2","alt":"H3K4me3 Antibody, SNAP-Certified™ for CUT&RUN"}],"date_added":"17th Jun 2020","pre_order":false,"show_cart_action":true,"has_options":false,"stock_level":null,"low_stock_level":null,"qty_in_cart":0,"custom_fields":[{"id":684,"name":"Pack Size","value":"100 µg"},{"id":685,"name":"Internal Comment","value":"extra box in top shelf of Venom"}],"num_reviews":null,"weight":{"formatted":"0.01 LBS","value":0.01},"demo":false,"add_to_cart_url":"https://www.epicypher.com/cart.php?action=add&product_id=760","price":{"without_tax":{"currency":"USD","formatted":"$525.00","value":525},"tax_label":"Sales Tax"},"add_to_wishlist_url":"/wishlist.php?action=add&product_id=760"}],"shipping_messages":[],"rating":0,"meta_keywords":"H3K4me1 antibody, histone H3K4me1 antibody, histone H3 lysine 4 monomethylated antibody, CUT&RUN antibody, CUT&Tag antibody","show_quantity_input":1,"title":"H3K4me1 Antibody, SNAP-Certified™ for CUT&RUN and CUT&Tag","gift_wrapping_available":false,"min_purchase_quantity":0,"customizations":[],"images":[{"data":"https://cdn11.bigcommerce.com/s-y9o92/images/stencil/{:size}/products/985/1115/snap-chip-ab__70507.1557259520.1280.1280__90586.1575483123.1280.1280__61997.1592491196__58196.1676557361__04967.1676559372.png?c=2","alt":"H3K4me1 Antibody, SNAP-Certified™ for CUT&RUN and CUT&Tag"}]}
- Type: Monoclonal [2088-1F4]
- Host: Rabbit
- Applications: CUT&RUN, CUT&Tag
- Reactivity: Human, Wide Range (Predicted)
- Format: Antigen affinity-purified
- Target Size: 15 kDa
Description
This H3K4me1 (histone H3 lysine 4 monomethyl) antibody meets EpiCypher’s lot-specific SNAP-Certified™ criteria for specificity and efficient target enrichment in both CUT&RUN and CUT&Tag applications. This requires <20% cross-reactivity to related histone PTMs determined using the SNAP-CUTANA™ K-MetStat Panel of spike-in controls (EpiCypher 19-1002, Figures 1 and 4). High target efficiency is confirmed by consistent genomic enrichment at varying cell inputs: 500k and 50k cells in CUT&RUN (Figures 2-3); 100k and 10k cells in CUT&Tag (Figures 5-6). High efficiency antibodies display similar peak structures at representative loci (Figures 3 and 6) and highly conserved genome-wide signal (Figures 2 and 5) even at reduced cell numbers. H3K4me1 either flanks H3K4me3 at the transcription start site (TSS) or coincides with H3K4me3 (Figures 2-3, 5-6) [1].
Validation Data - CUT&RUN
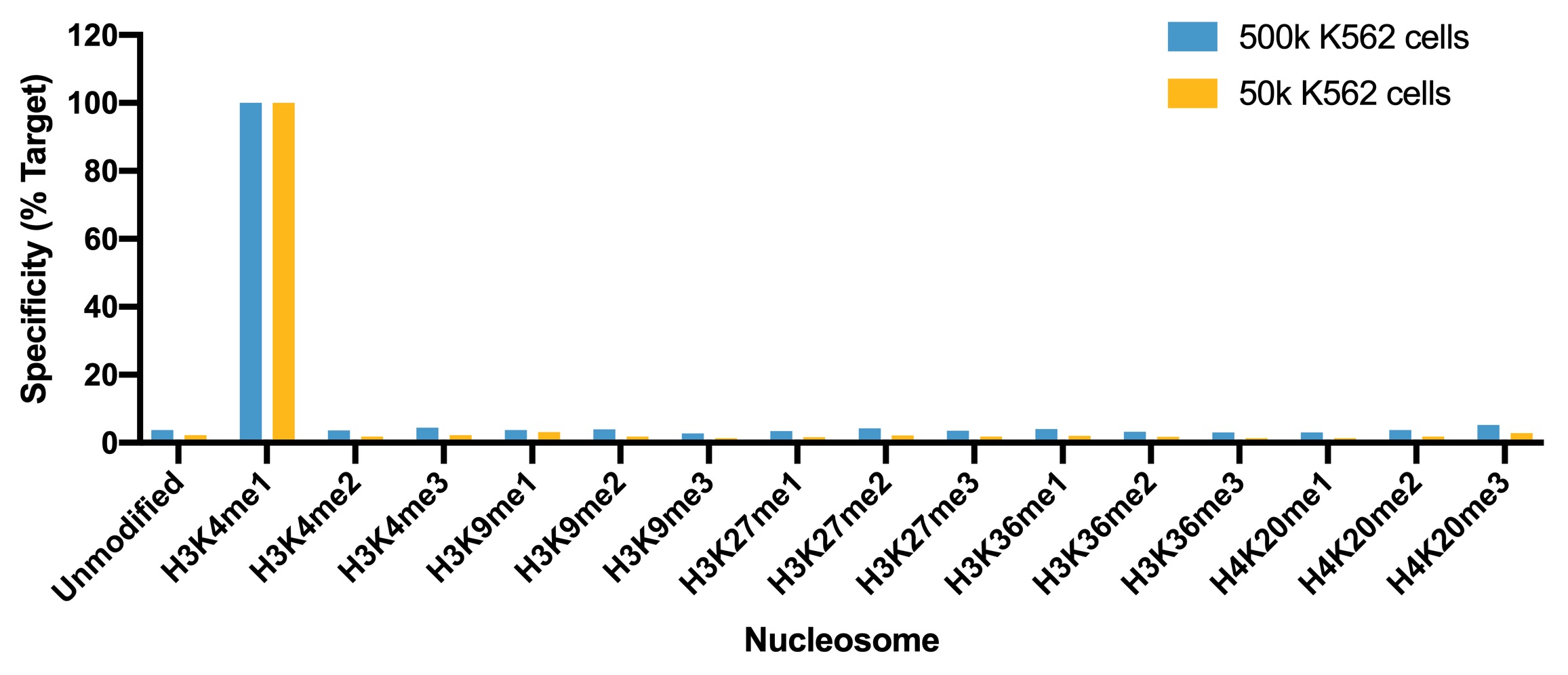
Figure 1: SNAP specificity analysis in CUT&RUN
CUT&RUN was performed as described above. CUT&RUN sequencing
reads were aligned to the unique DNA barcodes corresponding
to each nucleosome in the K-MetStat panel (x-axis). Data are
expressed as a percent relative to on-target recovery
(H3K4me1 set to 100%).
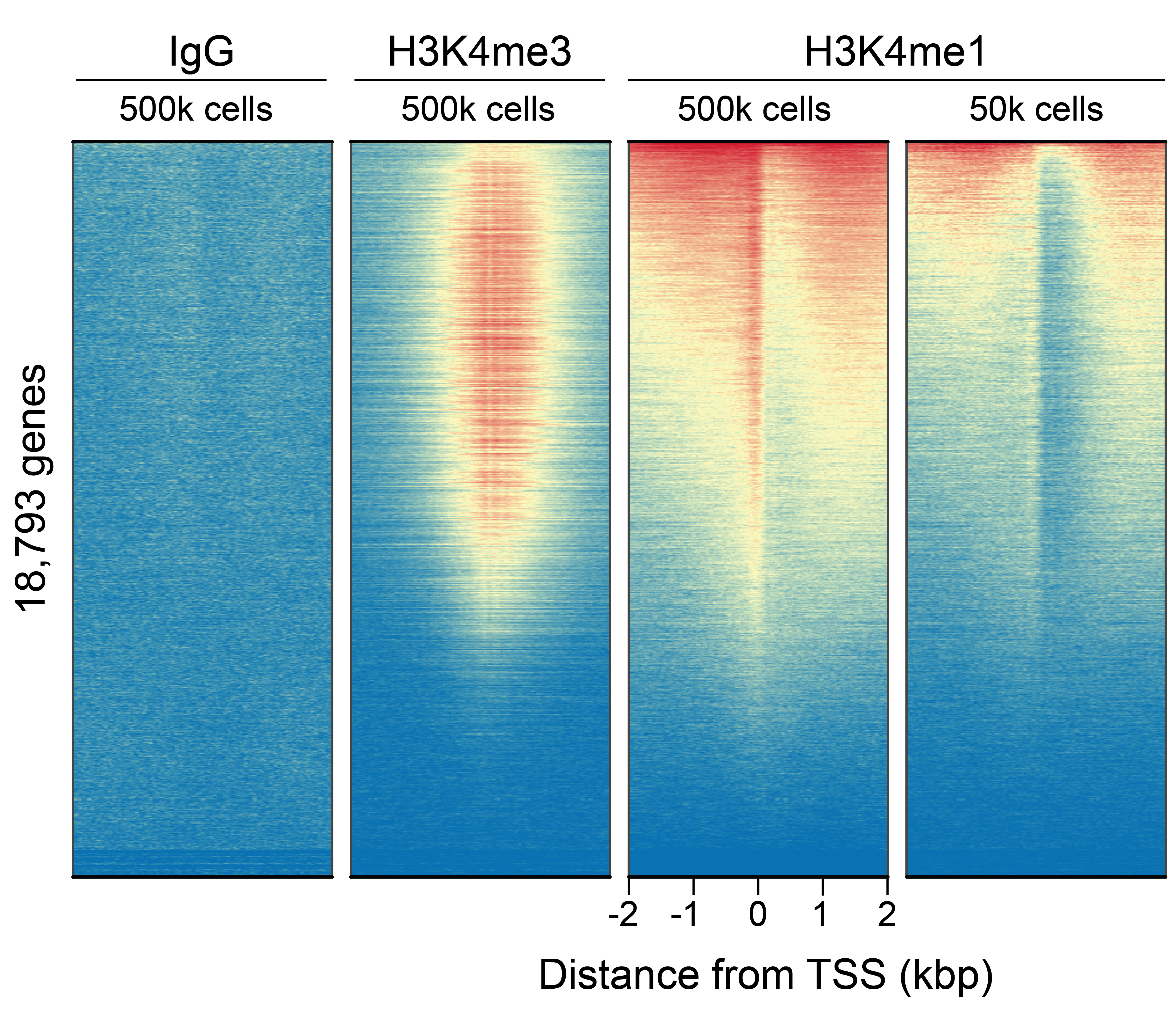
Figure 2: CUT&RUN genome-wide enrichment
CUT&RUN was performed as described above. Sequence reads
were aligned to 18,793 annotated transcription start sites
(TSSs ± 2 kbp). Signal enrichment was sorted from highest to
lowest (top to bottom) relative to the H3K4me1 - 500k cells
sample (all gene rows aligned). High, medium, and low
intensity are shown in red, yellow, and blue, respectively.
H3K4me3 positive control and H3K4me1 antibodies produced the
expected enrichment pattern, which was consistent between
500k and 50k cells and greater than the IgG negative
control.
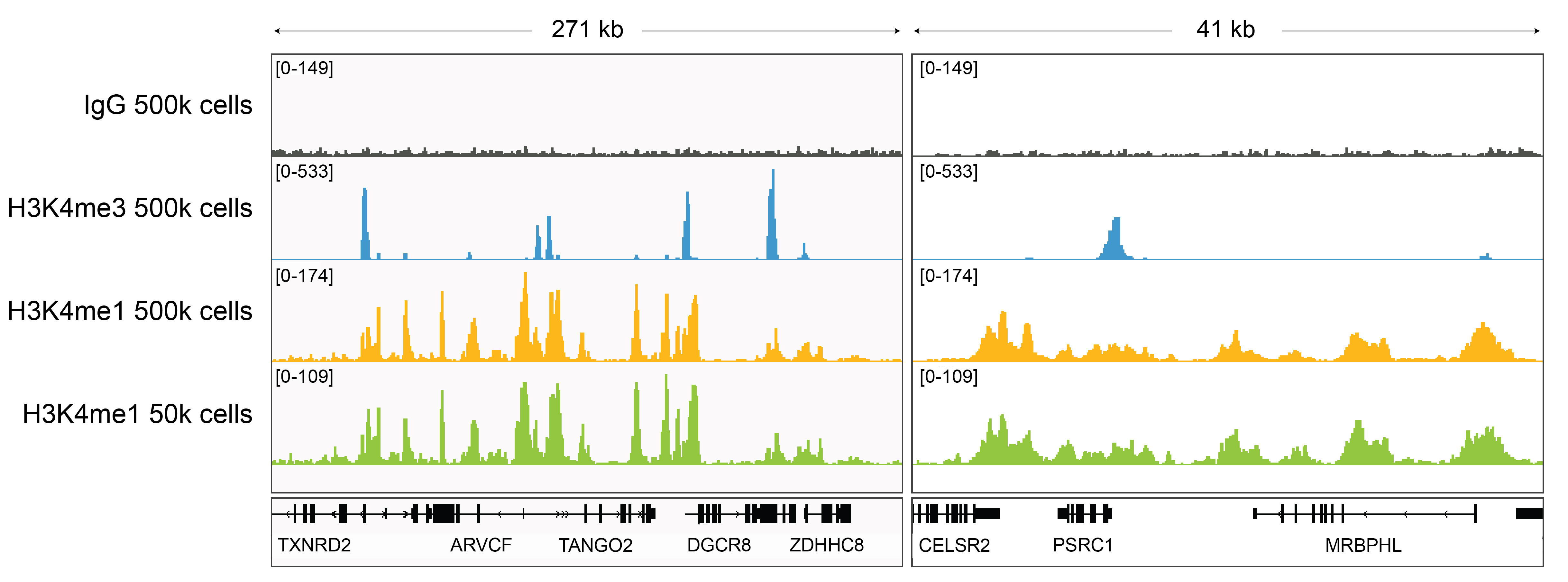
Figure 3: H3K4me1 CUT&RUN representative browser
tracks
CUT&RUN was performed as described above. Gene browser shots
were generated using the Integrative Genomics Viewer (IGV,
Broad Institute). H3K4me1 antibody tracks display the
characteristic enrichment known to be consistent with the
function of this PTM [1]. Similar results in peak structure
and location were observed for both 500k and 50k cell
inputs.
CUT&RUN methods
CUT&RUN was performed on 500k and 50k K562 cells with the
SNAP-CUTANA™ K-MetStat Panel (EpiCypher
19-1002) spiked-in prior to the addition of 0.5 µg of either IgG
negative control (EpiCypher
13-0042), H3K4me3 positive control (EpiCypher
13-0041), or H3K4me1 antibodies. The experiment was performed
using the CUTANA™ ChIC/CUT&RUN Kit v3.0 (EpiCypher
14-1048). Library preparation was performed with 5 ng of CUT&RUN
enriched DNA (or the total amount recovered if less than 5
ng) using the CUTANA™ CUT&RUN Library Prep Kit (EpiCypher
14-1001/14-1002). Both kit protocols were adapted for high throughput
Tecan liquid handling. Libraries were run on an Illumina
NextSeq2000 with paired-end sequencing (2x50 bp). Sample
sequencing depth was 6.7 million reads (IgG 50k cell input),
11.5 million reads (IgG 500k cell input), 10.2 million reads
(H3K4me3 50k cell input) and 16.7 million reads (H3K4me3
500k cell input). Data were aligned to the hg19 genome using
Bowtie2. Data were filtered to remove duplicates,
multi-aligned reads, and ENCODE DAC Exclusion List regions.
Validation Data - CUT&Tag
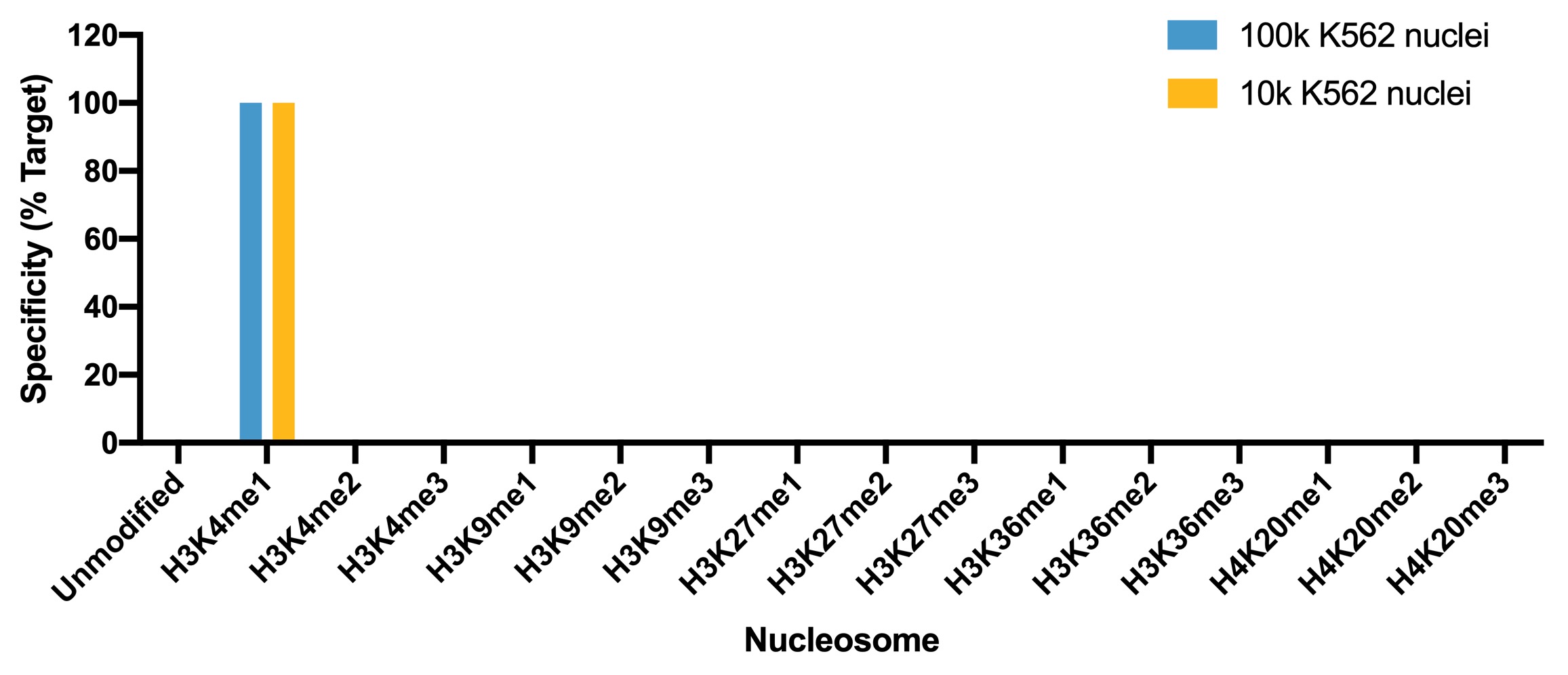
Figure 4: SNAP specificity analysis in CUT&Tag
CUT&Tag was performed as described above. CUT&Tag sequencing
reads were aligned to the unique DNA barcodes corresponding
to each nucleosome in the K-MetStat panel (x-axis). Data are
expressed as a percent relative to on-target recovery
(H3K4me1 set to 100%).
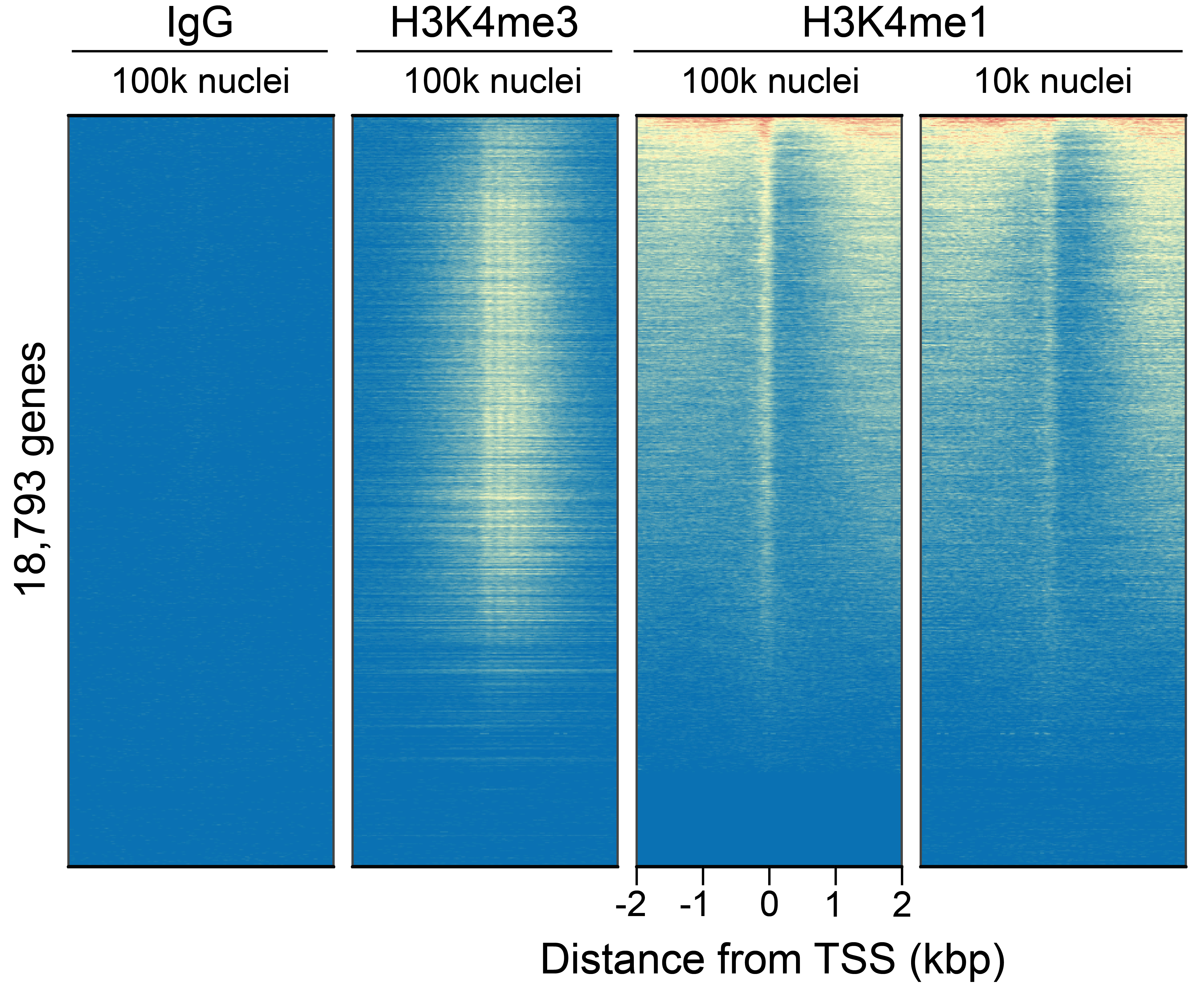
Figure 5: CUT&Tag genome-wide enrichment
CUT&Tag was performed as described above. Sequence reads
were aligned to 18,793 annotated transcription start sites
(TSSs ± 2 kbp). Signal enrichment was sorted from highest to
lowest (top to bottom) relative to the H3K4me1 - 100k nuclei
sample (all gene rows aligned). High, medium, and low
intensity are shown in red, yellow, and blue, respectively.
H3K4me3 positive control and H3K4me1 antibodies produced the
expected enrichment pattern, which was consistent between
100k and 10k nuclei and greater than the IgG negative
control.
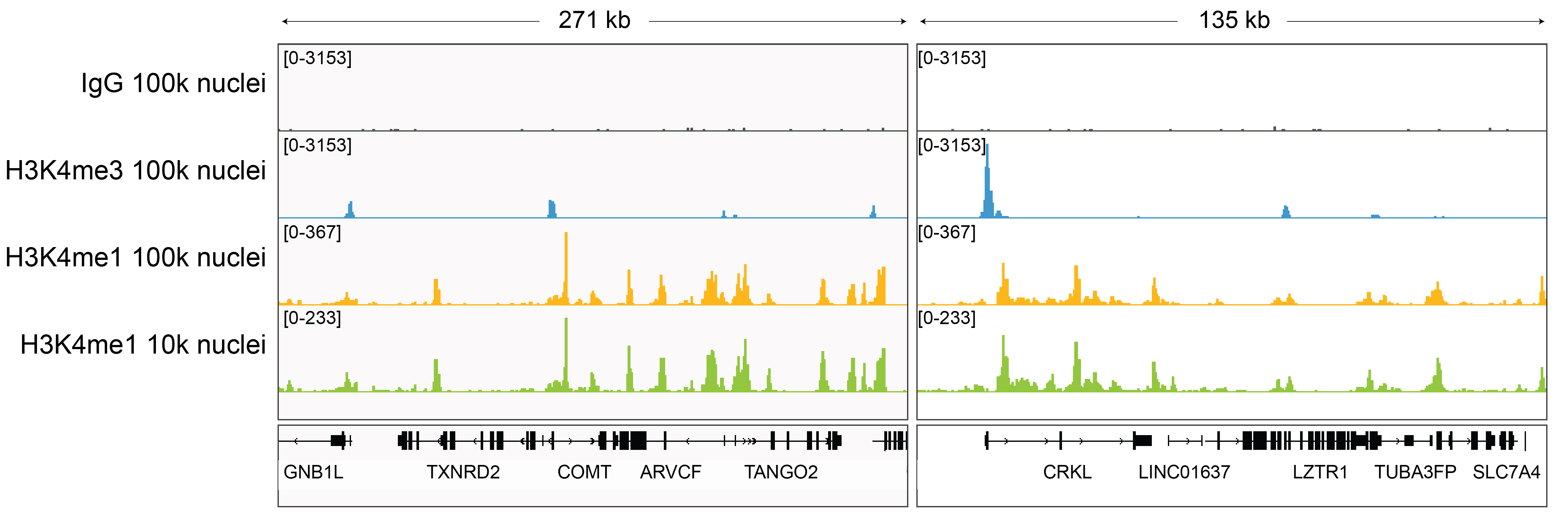
Figure 6: H3K4me1 CUT&Tag representative browser tracks
CUT&Tag was performed as described above. Gene browser shots
were generated using the Integrative Genomics Viewer (IGV,
Broad Institute). H3K4me1 antibody tracks display the
characteristic enrichment known to be consistent with the
function of this PTM [1]. Similar results in peak structure
and location were observed for both 100k and 10k nuclei
inputs.
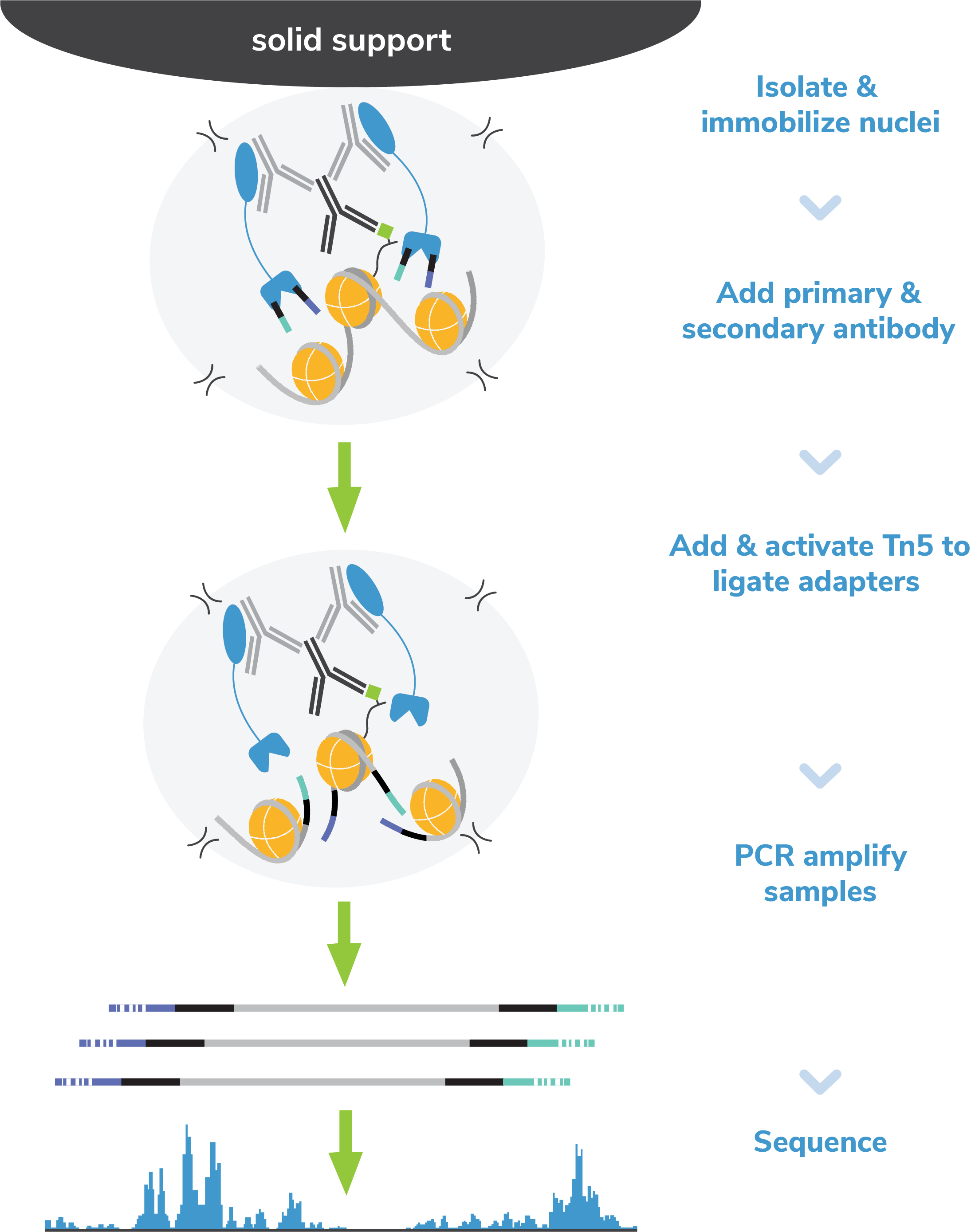
CUT&Tag methods
CUT&Tag was performed on 100k and 10k K562 nuclei with the
SNAP-CUTANA™ K-MetStat Panel (EpiCypher
19-1002) spiked-in prior to the addition of 0.5 µg of either IgG
negative control (EpiCypher
13-0042), H3K4me3 positive control (EpiCypher
13-0041), or H3K4me1 antibodies. The experiment was performed
using the CUTANA™ Direct-to-PCR CUT&Tag
Protocol. Libraries were run on an
Illumina NextSeq2000 with paired-end sequencing (2x50 bp).
Sample sequencing depth was 16.8 million reads (IgG 500k
cell input), 14.4 million reads (H3K4me3 500k cell input),
26.2 million reads (H3K4me1 500k cell input) and 11.4
million reads (H3K4me1 50k cell input). Data were aligned to
the hg19 genome using Bowtie2. Data were filtered to remove
duplicates, multi-aligned reads, and ENCODE DAC Exclusion List regions.